Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.3 La Habana jul.-set. 2013
RESEARCH
Expression and purification of a full-length recombinant NS1 protein from a dengue 2 serotype viral isolate
Expresión y purificación de la proteína completa NS1 del virus dengue serotipo 2 a partir de un aislamiento
Gilda Lemos, Isabel Guillén, Julio R Fernández, Tamara Díaz, Amanda B Colarte, María E Fernández de Cossío
Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba.
ABSTRACT
Dengue is an emerging disease that poses a threat to one-third of the global human population and produces over 50 million reported cases in tropical and sub-tropical regions every year. An accurate diagnosis of dengue infection is essential for timely management of the disease. NS1 is a 46- to 50-kilodalton highly conserved dengue virus glycoprotein that can be detected during the febrile phase of dengue virus (DENV) infection in both primary and secondary cases. This protein is a specific marker of DENV infection, and a sensitive test for NS1 would, if used together with IgM detection, provide an excellent diagnostic approach. Although the NS1 protein can be isolated from mammalian cell tissue cultures infected with DENV, this procedure is unsafe, laborious, and expensive and has very low yields, making it unsuitable for a large amount of antigen production. In this work, and with the objective of carrying out immunization experiments in mice, we cloned the full-length NS1 region from DENV serotype 2 (rNS1) in the vector pET28a with a 6xHis tag at the N-terminus. The protein was expressed in the Escherichia coli strain Rosetta as inclusion bodies, at the expected size of approximately 46 kDa, and further purified by metal-chelating affinity chromatography (IMAC) under denaturing conditions. Human sera from dengue positive cases showed reactivity to the recombinant NS1 protein by ELISA and Western blot. The unfolded rNS1 was directly used as immunogen. The polyclonal antibodies elicited in immunized mice with the recombinant antigen recognized the natural NS1 antigen from serotype 1 (sNS1).
Keywords: dengue virus, NS1, diagnosis, recombinant protein.
RESUMEN
El diagnóstico certero de la infección por el virus dengue (DENV) es esencial para su tratamiento oportuno. La proteína NS1 (46-50 kDa) es una glicoproteína del DENV de secuencia altamente conservada en los cuatro serotipos del virus, que se puede detectar durante la fase febril del dengue en pacientes infectados por primera o segunda vez, como marcador específico de la infección. Por ello, un test basado en la proteína NS1, pudiera facilitar su diagnóstico cuando se emplea junto con la detección de la inmunoglobulina M (IgM) contra el DENV. Entre las principales dificultades para su obtención está el aislamiento de la proteína NS1 de cultivos celulares de mamíferos, lo cual no es seguro, es laborioso y caro, y con bajos rendimientos que impiden su escalado. En este trabajo se clonó la secuencia completa de la proteína NS1 (rNS1) del DENV serotipo 2 en el vector pET28a, fusionado con una cola de histidina 6xHis en el extremo N-terminal. Esta proteína se obtuvo de forma recombinante en Escherichia coli, cepa Rosetta, como cuerpos de inclusión, con aproximadamente 46 kDa, y se purificó por cromatografía de afinidad de quelatos metálicos (IMAC) en condiciones desnaturalizantes. Sueros humanos de pacientes positivos al dengue mostraron reactividad contra la rNS1 en ensayos de ELISA y Western blot. La proteína rNS1 desnaturalizada se administró directamente como inmunógeno en ratones Balb/C, cuya respuesta de anticuerpos policlonales detectó a la proteína NS1 natural del DENV serotipo 1 en ensayos de inmunoblot.
Palabras clave: virus dengue, NS1, diagnóstico, proteína recombinante.
INTRODUCTION
Dengue is an arthropod-borne viral disease and has been a major cause of morbidity and mortality in recent decades. Dengue virus (DENV) is considered one of the most important emerging viruses, posing a threat to one-third of the global human population, with over 50 millions of cases reported in tropical and sub-tropical regions every year [1]. Most infections are asymptomatic, and symptomatic cases exhibit a wide range of clinical manifestations, being the most common outcome an acute febrile illness similar to influenza (dengue fever, DF). However, in a minority of cases, this progresses to spontaneous hemorrhaging (dengue hemorrhagic fever, DHF) and, most seriously, to dengue shock syndrome (DSS), characterized by circulatory failure. There are perhaps 500 000 cases of DHF/DSS each year, with case-fatality rates as high as 5 % depending on the availability of treatment [2].
Dengue is caused by one to four dengue serotypes (DENV type 1 through 4), of the genus flavivirus (family Flaviviridae).The viral agent is a single-stranded, positive-sense, RNA virus with a genome of approximately 11 kb.
Co- and post-translational processing gives rise to three structural and seven nonstructural proteins: C, prM, E, NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5. Antigenic diversity of the DENV is important, since the lack of long-term cross-immunity among the four virus types allows for multiple sequential infections [3].
NS1 is a 46- to 50-kilodalton highly conserved glycoprotein that is expressed in both membrane-associated (mNS1) and secreted (sNS1) forms [4, 5] and possesses both group-specific and type-specific determinants [6, 7]. NS1 is an atypical viral glycoprotein because it does not form part of the virion structure but is expressed on the surface of infected cells. While the function of NS1 is yet to be fully defined, preliminary evidence has shown it to be involved in viral RNA replication [8, 9].
More effective and rapid diagnosis can contribute to the control of dengue and DHF through more accurate public health notification. Many reports suggested the use of NS1 detection for early diagnosis of dengue infection in primary and secondary cases, period in which dengue antibodies are still undetectable [10-12], since high concentrations of the NS1 protein varying from 0.04 to 2 µg/mL in acute-phase serum samples to only 0.04 µg/mL or even less in convalescent phase serum [10] were found in blood samples of patients obtained during the early acute phase of both primary and secondary DENV infections and for up to 9 days after the onset of symptoms. Levels of NS1 antigen remain detectable even in some cases when viral RNA is negative by reverse transcriptase-PCR.
Early detection of NS1 antigen during the febrile stage of the disease combined with IgM detection should expand the time span during which a rapid test could detect a dengue infection and make it a sensitive diagnostic approach.
Methods for isolating the natural NS1 antigen from mammalian cells infected with DENV yield only low amounts of protein, since secreted antigen concentration ranges from 5 to 10 µg/mL culture supernatant [13]. Therefore, using recombinant NS1 protein for immunizing mice for hybridoma production, or as a diagnostic antigen for dengue viral infection, is a more suitable approach. Several works have reported the successful use of heterologous expression systems such as baculovirus [14] and Pichia pastoris [15] for the expression of the NS1. The Escherichia coli bacterial expression system has been also widely exploited for NS1 expression, although it always involves refolding procedures, facilitating disulfide bond formation and thus renaturation of the protein [8, 16-19].
In this work, we cloned and expressed the full length NS1 region from serotype 2 in E. coli and the denatured antigen was directly used as immunogen. The antibodies obtained in immunized mice will recognize only linear epitopes in the natural secreted antigen, but several reports have shown the immune-dominancy and cross-reactivity of linear epitopes present in NS1 protein from all four DENV serotypes [7, 13, 20, 21]. The polyclonal antiserum from animals immunized with the denatured recombinant NS1 protein from serotype 2 (rNS1) was found to specifically recognize the natural NS1 antigen from serotype 1, thus demonstrating the possibility of generating antibodies that recognize epitopes in the native NS1 antigen from a different serotype using the unfolded antigen.
MATERIALS AND METHODS
Human serum samples
A panel of sera from 11 dengue patients (5 samples from the acute disease stage and 6 samples from convalescent-phase of the disease) and 14 healthy human sera were used in the study. Dengue positive serum samples used in this study were confirmed from dengue infected patients collected during an epidemic DENV-4 outbreak in Havana city in 2006. DENV infections were defined as febrile illness associated with the detection of virus specific IgM (UMELISA® IgG and IgM antibody detection kits, TecnoSuma®, Havana, Cuba). Serum samples were collected between days 3 and 30 after the onset of symptoms. Convalescent-phase sera refer to specimens collected during days 7 to 30 days since onset of fever. Healthy human sera sample refers to sera collected from healthy blood donors.
Viral RNA isolation and purification from Dengue Virus Mammalian Cell Culture
Supernatant from Vero cells (10 mL) infected with 106 pfu/mL of SB8553 DENV-2 viral strain (kindly provided by Dr MJ Cardosa, University Sarawak, Malaysia) was harvested 144 hh post-inoculation without media changing. Viral particles were concentrated by centrifugation at 20 000 × g for 1 h at 4 °C after clarification using a solution of 4 % PEG8000 plus 0.5 M NaCl for 4 hours at 4 °C. RNA was purified from precipitated viral particles using the Ambion Tri-Reagent procedure for suspension cells. Briefly, the pellet was resuspended in 5 mL of Tri-Reagent (Sigma-Aldrich, St. Louis, USA) by vigorous vortex mixing and pipetting. After 5-min incubation at 20 °C, each sample was transferred to a 15-mL polypropylene centrifuge tube and 1.2 mL of chloroform was added. Samples were vigorously vortexed for 30 s, incubated at room temperature for 5 min and centrifuged at 12 000 × g for 5 min. The top aqueous layer, containing the RNA, was precipitated by adding 0.5 volumes (approximately 2.5 mL) of isopropanol for 5 min at 20 °C, followed by centrifuging at 5000 × g for 5 min. The resulting white pellets were washed with cold 75 % ethanol, and each pellet was then resuspended in 300 μL of RNase-free water.
Cloning of dengue NS1 full-length viral protein
cDNA was obtained from 2 µg of total RNA by using an RT-PCR Kit from Promega M-MLV procedure (Part# 9PIM170, USA). For PCR procedure, the forward primer 5´-GCGGATCCATGAATTCAC GCAGCACCTC-3´ and the reverse 5´-GCCTCGAGCT G GCTGTGACCAAGGAGT-3´, with BamH I and Xho I sites included (bold letters) were used. The PCR-amplified region was inserted in the pGEM®-T Easy Vector (Promega, USA) and the BamH I-Xho I-NS1 region was further inserted in the pET28a (+) expression vector (Novagen, Darmstadt, Germany), generating the recombinant plasmid pET28a-NS1, which was transformed subsequently in chemically competent E. coli DH5α cells. Plasmid DNA samples from recombinant bacterial colonies were analyzed by digestion with BamH I and Xho I, PCR and DNA sequencing (Macrogen, Korea) coupled to BLAST (Basic Local Alignment Search Tool, NCBI), performed using the M13F-pUC/SP6 and T7 promoter/T7 terminator primers for vectors pGEM®-T Easy Vector and pET28a (+), respectively. The expressed protein was predicted to have an isotopically averaged molecular weight of approximately 46.4 kDa, corresponding to 380 amino acids of the NS1 protein and 34 amino acids encoded by the expression vector, including the N-terminal 6xHis-tag.
Expression and purification of the rNS1 antigen
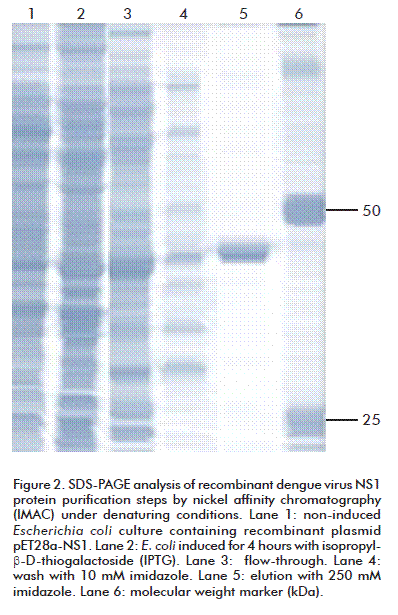
Expression experiments of the recombinant protein were performed by the induction of the pET System E. coli host Strains: BL21 (DE3), Tuner (DE3) and Rosetta (DE3), cultured in 300 mL LB broth (containing 50 µg/mL kanamycin) in a 1000 mL conical flask and cultured at 37 °C in a shaker set at 200 rpm and induced by adding IPTG to a final concentration of 1 mM at 0.5-1.0 optical density (O.D.) at 600 nm. Cultures were harvested 3-4 hours after induction and E. coli cells were obtained by centrifugation at 3 000 × g for 30 min at 4 °C. After discarding the supernatant, 1 g of wet bio-mass was resuspended in 10 mL of TE 1 (10 mM Tris-HCl pH 8.0 and 5 mM EDTA) and disrupted by three passes in French press at a pressure of 100 bars at 4 °C. The obtained lysate was centrifuged at 20 000 × g for 20 min at 4 °C. Insoluble proteins were solubilized in 10 mL of Buffer A: 10 mM Tris-HCl, 100 mM NaH2PO4, 10 mM β-mercaptoethanol and 8 M urea, for 1 h at 4 °C, obtaining the soluble proteins fractions by centrifugation at 20 000 × g for 20 min at °4 C and NS1 6xHis-tagged protein was further purified by affinity chromatography using 3 mL of Ni2+-nitrilotriacetic acid (Ni-NTA) resin (Qiagen, Germany). Washed and elution steps were performed using 10 and 250 mM Imidazole in buffer A. Protein concentration was determined by the bicinchoninic acid assay (BCA, Pierce/ThermoFisher, Rockford, USA) and the purified antigen aliquots were stored at -20 °C until use. Protein expression level and purity were assessed by densitometric analysis of the sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Purification of monoclonal antibody 15F3-1
Ascites fluid containing 15F3-1, an anti-NS1 Mab (ATCC HB-47), was extracted from mice and diluted 1:4 in phosphate buffer saline (PBS), purifying the antibody by chromatography on a Protein A-Sepharose column. Purified 15F3-1 Mab was further coupled to CNBr-activated Sepharose 4 Fast Flow (Amersham/GE Healthcare, USA), according to the manufacturer’s procedure.
Purification of NS1 natural antigen of dengue serotype 1 from Vero cell culture supernatant
The natural NS1 antigen from dengue serotype 1 was purified by affinity chromatography on 15F3-1-Sepharose using a method described by Young et al. [10]. Briefly, 350 mL of supernatant from Vero cells infected previously with 106 pfu/mL of DENV-1 West Pac 74 (NIBSC) and harvested after a period of 144 hours post-inoculation without changing the medium were clarified as described above and passed through 15F3-1-Sepharose, equilibrated with TNE buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl and 5 mM EDTA). After washing the column with TNE buffer, sNS1 was eluted in TNE containing 40 mM diethylamine. Protein concentration was determined by BCA (Pierce/ThermoFisher) and purified antigen aliquots were stored at -20 °C.
Mouse immunizations
Balb/c (Bc-H-2d) female mice (aged 6-8 weeks), 16-18 g of weight, purchased from Cenpalab (Havana, Cuba) were inoculated using two different procedures. Group 1 (5 animals) was inoculated subcutaneously using 100 µL of the immunogenic preparation (20 µg of rNS1 per dose) using Freund’s complete adjuvant (Sigma-Aldrich, USA) in the first dose and incomplete adjuvant in subsequent doses. For Group 2 (5 animals), the first two doses were performed as for Group 1 but subsequently the animals were administered intraperitoneally (i.p.) with 200 µL of the immunogen preparation in PBS. Both groups were immunized at 15-day intervals up to a total of 5 immunizations.
ELISA procedures
Costar 3591 plates were coated with 100 µL of 0.25 µg per well of rNS1 or 2 µg per well of natural DENV-1 sNS1 antigen in Coating Buffer (0.1 M carbonate/bicarbonate buffer pH 9.6). The plates were incubated for 1 hour at 37 °C. After washing three times with distilled water and 0.05 % Tween-20, the coated plates were blocked with 200 µL per well of blocking solution (PBS, 5 % skim milk powder, 0.05 % Tween-20) for one hour at 37 °C. Blocking solution was discarded by tapping the plate and 100 µL of test sample, at desired dilution in PBS, 0.5 % skim milk powder, 0.05 % Tween-20, were added per well and incubated for 1 hour at 37 °C. Bound specific antiserum was detected using 100 µL per well of a secondary antibody (1/10000 dilution of mouse (Fc specific) goat peroxidase-conjugated (Cat. # A2554, Sigma-Aldrich, USA, for mouse samples) and anti-human IgG goat peroxidase-conjugate (Cat. # A0170, Sigma-Aldrich, USA) for human samples, diluted in PBS-T solution (2.68 mM KCl, 1.47 mM KH2PO4, 136.89 mM NaCl, 8.1 mM Na2HPO4 and 0.05 % Tween-20). After washing, the color reaction was developed using 5 mg of o-Phenylenediamine dihydrochloride (OPD, Cat #P6912, Sigma-Aldrich, USA) as chromogen and 5 µL of 30 % hydrogen peroxide as substrate (Caledon, Canada). The reaction was stopped with a solution of 2 M H2SO4. Absorbance (O.D.) was read at 492 nm using a UMELISA® reader (PR-521, Tecnosuma Internacional, Cuba). The cut-off O.D. for testing the seropositivity of each sample was defined as an adjusted O.D. 492 nm of the mean plus 2 standard deviations of the negative control sera. For mouse serum samples, a non-immune mouse serum was used as a negative control and a pool of sera from mice immunized with the recombinant antigen was used as a positive control. For human ELISA assays, a serum pool from healthy individual was used as a negative control and serum sample from a dengue convalescent confirmed by serum IgM assay was used as a positive control.
Immunoblot analysis
Purified rNS1 expressed in E. coli was analyzed for its reactivity to DENV-specific antibodies present in mouse and human sera by Western blot. Briefly, samples from cell extracts and purified 6xHis-rNS1 were separated by 12.5 % SDS-PAGE [22], and either stained with Coomassie brilliant blue R250 (Sigma-Aldrich) or electro-transferred using a Trans-Blot® SD Semi-Dry Electrophoretic Transfer Cell (BioRad, USA) to a nitrocellulose membrane (Amersham/GE Healthcare, USA) for 30 min in transfer buffer (48 mM Tris pH 9.0-9.4, 39 mM Glycine, 20 % methanol and 1.3 mM SDS). The membrane was blocked for 1 hour at 37 °C with blocking buffer (PBS-T and 5 % skim milk powder, BDH, UK). Human serum samples were diluted 1:20 with sample buffer (PBS, 0.25 % non-fat skim milk powder and 0.05 % Tween-20) and incubated with the nitrocellulose membranes for one hour at 37 oC. After incubation, the membranes were washed three times, 5 min each, with PBS and 0.05 % Tween-20 solution. After washing, the membrane was allowed to react with a 1:1000 dilution of an anti-Human IgG (Fc specific)-peroxidase antibody produced in goat (Cat. # A0170, Sigma-Aldrich, USA) for detecting human antibodies or an Anti-mouse (Fc specific)-peroxidase antibody at the same dilution (Sigma-Aldrich, USA), for detecting mouse antibodies, for 1 h at 37 ºC. After a washing step, immunoreactivity was detected using 3, 3’-Diaminobenzidine tetra hydrochloride hydrate (DAB, Cat. # D5637, Sigma-Aldrich, USA) in 10 mL PBS and 0.05 % (v/v) H2O2. The reaction was stopped by washing several times with distilled water.
Commercially available recombinant protein NS1 from serotype 2 (Nterminal-rNS1; Cat #. 00342-V, Virogen, Boston, MA) was used as a control protein in immunoblotting assays.
Statistical analysis
The statistical significance of immunological differences among human serum samples and the analysis of immunized mouse groups were performed by unpaired Student’s t-test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Cloning, expression and purification of DENV-2 rNS1 protein
The NS1 full-length gene was cloned with the 6xHis tag at the N-terminus in vector pET28a (+). Blast analysis of the cloned fragment (1139 bp) showed that the sequence matched to 99 % the sequence available for NS1 from a serotype 2 Malaysian isolate at GenBank (GenBank accession number FN429892.1).
In the expression experiments carried out with different E. coli strains, the Tuner and the Rosetta strains exhibited the best results (Figure 1). The rNS1 protein was expressed at the expected size of approximately 46 kDa, co-migrating with an E. coli protein as visualized in SDS-PAGE. A Western blot probed with mouse anti-NS1 polyclonal antibody showed some smaller sized bands in both strains. Therefore, further expression experiments of the rNS1 protein were carried out with the Rosetta strain. The best yields of wet biomass and antigen expression were obtained inducing the culture with IPTG when O.D. 600 nm was 1.0 and harvesting 4 hours after induction (results not shown). The rNS1 expression level in Rosetta E. coli strain was approximately 5 % by densitometric analysis of the SDS-PAGE.
The rNS1 protein, predominantly expressed in E. coli as inclusion bodies, was isolated from bacterial shake flask culture and purified by IMAC chromatography under denaturing conditions (Figure 2). During the initial purification process by Ni-NTA columns, more than 20 % of the NS1 protein eluted during the 10 mM imidazole wash step. Then, for better protein recovery, 10 mM beta-mercaptoethanol was added to solubilization and chromatography purification buffers. IMAC purification under denaturing conditions yielded significant amount of more than 87 % pure rNS1 protein, with a single band as judged by SDS-PAGE (Figure 2). The rNS1 produced by E. coli Rosetta (DE3) was 13.1 mg/L. Purification steps showed a typical yield of 60-70 % of starting denatured inclusion bodies (Table).
Purification of DENV-1 sNS1 from Vero cell supernatant
Secreted DENV-1 NS1 protein was successfully purified from dengue-infected Vero cells culture supernatants by immunoaffinity chromatography, secreted NS1 usually reaches concentrations ranging from 5 to 10 µg/mL [23]. In this work, we obtained approximately 7 µg of antigen per milliliter of culture medium supernatant harvested at 144 h post-inoculation.
Immunological characterization of DENV-2 rNS1
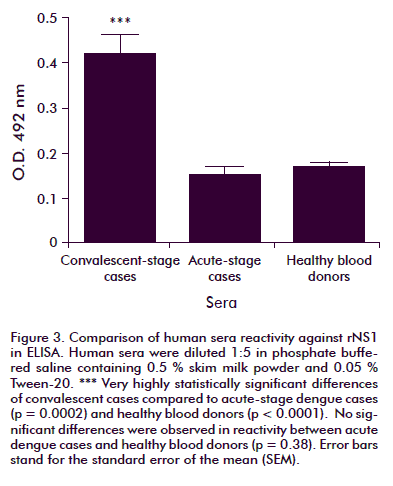
The reactivity of human sera against rNS1 was established by ELISA and Western blot. The ELISA test results are shown in figure 3. From dengue-positive samples, only the six convalescent-stage cases exhibited reactivity against the antigen, a statistically significant difference compared to the reactivity exhibited by samples from the acute stage of the disease (p = 0.0002) or from healthy donors (p < 0.0001). No significant difference in reactivity was observed between acute cases and healthy blood donors (p = 0.38).
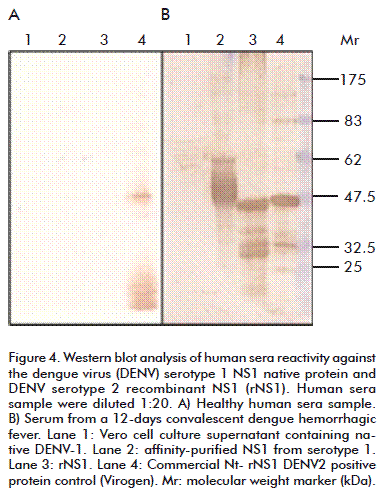
A serum sample from a 12 days convalescent severe DHF female case showed the highest reactivity. Western blot analysis of rNS1 (Figure 4) showed that human dengue convalescent patient sera bound the obtained rNS1 at least as well as it did with Virogen serotype 2 NS1, but in both cases, smaller sized bands were also recognized by positive human sera.
Mouse immunization results
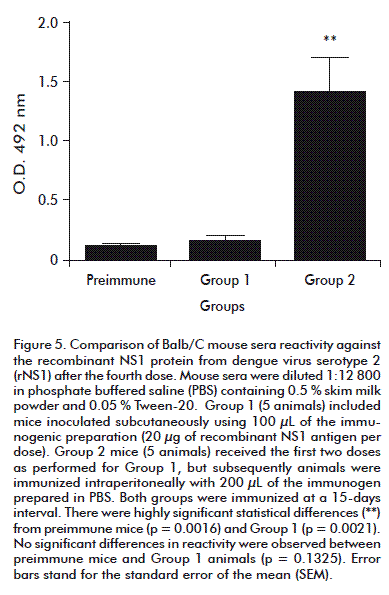
Mice immunized with the recombinant antigen showed high reactivity against rNS1 in ELISA, but reactivity depended on inoculation route. After the fourth dose (Figure 5) Group 2 (mice inoculated by i.p. route) exhibited significantly higher levels of anti-rNS1 antibodies than mice immunized subcutaneously (p = 0.0009), prompting a switch to i.p. administration for both groups.
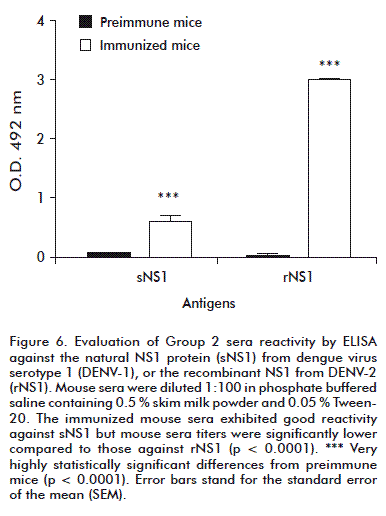
After the fifth and last dose, when comparing reactivity against the natural or recombinant antigens in ELISA, the immunized mice sera from Group 2 exhibited good reactivity against natural DENV-1 sNS1 (Figure 6), although their titers were significantly lower compared with those against DENV-2 rNS1 (p < 0.0001).
DISCUSSION
Previous reports have described different heterologous protein expression systems, such as baculovirus, yeast expression systems and vaccinia virus, for producing secreted and glycosylated recombinant DENV NS1 protein [24-29]. Several reports have also proposed achieving NS1 expression using bacterial cells [11, 16-19] since prokaryotic systems are easy to manipulate and express high levels of recombinant proteins. The inconvenience of these methods is that the expressed antigen lacks posttranslational modifications; therefore, refolding protocols must be used to produce rNS1 with a native-like protein conformation, preserving important conformation and antigenic determinants of the natural virus protein, not present in the denatured form of the antigen [14, 18, 30, 31]. However, these protocols are time consuming and sometimes very inefficient.
This study was aimed at obtaining a serotype 2 NS1 recombinant antigen useful for mouse immunizations with the objective of generating hyper-immune mouse sera against the viral antigen. In our work, the strategy was to use the denatured rNS1 protein as immunogen. Antibodies obtained with the denatured recombinant protein will recognize only linear epitopes in the natural secreted antigen, but it has been reported that linear epitopes, present in NS1 protein, are immune-dominant and cross-reactive to all four DENV serotypes [7, 13, 20, 21].
Expression experiments showed similar results using Rosetta and Turner E. coli strains (Figure 1) but additional smaller sized bands were observed in immunoblotting experiments, perhaps due to truncated NS1 protein or degradation. Although a moderate expression level (5 %) was obtained, we decided to continue using Rosetta strain, mainly because of the high percentages of rare codons for this host such as AGA, CCA and CAC in the cloned insert (11 %). This ‘non-optimal gene’ could reduce the efficiency of translation or even disengage the translational machinery. The Rosetta strain enhances expression of proteins having codons rarely used in E. coli; by supplying tRNAs for six rare codons: AUA, AGG, AGA, CUA, CCC, and GGA. In the case that higher yield of the protein devoid of degradation products or truncated protein is required, an additional purification step could be used, to discriminate degradation products by molecular size (e.g., size exclusion chromatography), since the affinity process captures all the molecules bearing the histidine tag, and particularly small fragments based on their lower steric hindrance. Additionally, conditions more restrictive for degradation could be also implemented during sample rupture.
For better protein recovery during the purification process by Ni-NTA, 10 mM beta-mercaptoethanol was added to the solubilization and chromatography purification buffers. The viral protein sequence contains 12 cysteine residues and some folded structures could therefore be present even when using denaturants such as 8 M urea, hindering proper exposure of the 6xHis tag.
The purified recombinant antigen was recognized in Western blot analysis using dengue convalescent-phase sera, but results showed several smaller sized bands, as explained above, perhaps due to incomplete protein expression, degradation or perhaps anomalous electrophoretic behavior of the NS1 protein [30].
In the ELISA assay, 6 samples from convalescent-phase disease showed reactivity against the denatured recombinant antigen. The highest O.D. was obtained with a 12 days convalescent severe DHF female case. Some human dengue-infected patient samples exhibited no or poor reactivity against the recombinant antigen, but all of them were samples from the acute stage of the disease, in which antibody titers against the viral antigen might be still low.
The unfolded rNS1 antigen was used as immunogen and hyper-immune mice sera against the viral NS1 antigen were obtained. In this paper, it is shown that the antibodies elicited in immunized mice with the denatured DENV-2 rNS1 protein have good reactivity against the secreted NS1 protein from DENV-1. This antigen therefore could be useful for obtaining specific antibodies against the natural viral proteins; possibly to conserved regions of the four serotypes, for example amino acids residues 154-161 which are highly conserved among different DENV serotypes [32].
This approach could be useful for obtaining monoclonal antibodies that could recognize secreted NS1 present in the sera of infected dengue patients, enabling the development of an NS1 antigen capture test for an early and accurate detection of acute infection in suspected dengue cases.
REFERENCES
1. Holmes EC, Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol. 2003;3(1):19-28.
2. World Health Organization. Strengthening implementation of the global strategy for Dengue Fever and Dengue Haemorrhagic Fever, prevention and control. Report of the Informal Consultation, Geneva: WHO HQ; 18-20 October 1999. Available from: http://apps.who.int/iris/handle/10665/66186.
3. Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366(15):1423-32.
4. Crooks AJ, Lee JM, Easterbrook LM, Timofeev AV, Stephenson JR. The NS1 protein of tick-borne encephalitis virus forms multimeric species upon secretion from the host cell. J Gen Virol. 1994;75(Pt 12):3453-60.
5. Talarmin A, Labeau B, Lelarge J, Sarthou JL. Immunoglobulin A-specific capture enzyme-linked immunosorbent assay for diagnosis of dengue fever. J Clin Microbiol. 1998;36(5):1189-92.
6. Kuno G, Vorndam AV, Gubler DJ, Gomez I. Study of anti-dengue NS1 antibody by western blot. J Med Virol. 1990;32(2):102-8.
7. Falconar AK, Young PR, Miles MA. Precise location of sequential dengue virus sub complex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch Virol. 1994;137(3-4):315-26.
8. Mason PW. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology. 1989;169(2):354-64.
9. Markoff LJ, Innis BL, Houghten R, Henchal LS. Development of cross-reactive antibodies to plasminogen during the immune response to dengue virus infection. J Infect Dis. 1990;164(2):294-301.
10. Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol. 2000;38(3):1053-7.
11. Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40(2):376-81.
12. Shu PY, Chen LK, Chang SF, Su CL, Chien LJ, Chin C, et al. Dengue virus serotyping based on envelope and membrane and nonstructural protein NS1 serotype-specific capture immunoglobulin M enzyme-linked immunosorbent assays. J Clin Microbiol. 2003;42(6):2489-94.
13. Falconar AK, Young PR. Immunoaffinity purification of native dimer forms of the flavivirus non-structural glycoprotein NS1. J Virol Methods. 1990;30(3):323-32.
14. Qu X, Chen W, Maguire T, Austin F. Immunoreactivity and protective effects in mice of a recombinant dengue 2 Tonga virus NS1 protein produced in a baculovirus expression system. J Gen Virol. 1993;74(Pt 1):89-97.
15. Zhou JM, Tang YX, Fang DY, Zhou JJ, Liang Y, Guo HY, et al. Secreted expression and purification of dengue 2 virus full-length nonstructural glycoprotein NS1 in Pichia pastoris. Virus Genes. 2006;33(1):27-32.
16. Xu H, Di B, Pan YX, Qiu LW, Wang YD, Hao W, et al. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: implications for early diagnosis and serotyping of Dengue virus infections. J Clin Microbiol. 2006;44(8):2872-8.
17. Qiu LW, Di B, Wen K, Wang XS, Liang WH, Wang YD, et al. Development of an antigen capture immunoassay based on monoclonal antibodies specific for Dengue virus serotype 2 nonstructural protein 1 for early and rapid identification of Dengue virus serotype 2 infections. Clin Vaccine Immunol. 2009;16(1):88-95.
18. Das D, Mongkolaungkoon S, Suresh MR. Super induction of dengue virus NS1 protein in E. coli. Protein Expr Purif. 2009;66(1):66-72.
19. Allonso D, da Silva Rosa M, Coelho DR, da Costa SM, Nogueira RM, Bozza FA, et al. Polyclonal antibodies against properly folded Dengue virus NS1 protein expressed in E. coli enable sensitive and early dengue diagnosis. J Virol Methods. 2011;175:109-16.
20. Huang JH, Wey JJ, Sun YC, Chin C, Chien LJ, Wu YC. Antibody responses to an immunodominant nonstructural 1 synthetic peptide in patients with dengue fever and dengue hemorrhagic fever. J Med Virol. 1999;57(1):1-8.
21. Chen Y, Pan Y, Guo Y, Qiu L, Ding X, Che X. Comprehensive mapping of immunodominant and conserved serotype- and group-specific B-cell epitopes of nonstructural protein 1 from dengue virus type 1. Virology. 2010;398(2):290-8.
22. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680-5.
23. Flamand M, Megret F, Mathieu M, Lepault J, Rey FA, Deubel V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J Virol. 1999;73(7):6104-10.
24. Falgout B, Chanock R, Lai CJ. Proper processing of Dengue virus nonstructural glycoprotein NS1 requires the N-terminal hydrophobic signal sequence and the downstream nonstructural protein NS2a. J Virol. 1989;63(5):1852-60.
25. Pryor MJ, Wright PJ. The effects of site-directed mutagenesis on the dimerization and secretion of the NS1 protein specified by Dengue virus. Virology. 1993;194(2):769-80.
26. Falgout B, Markoff L. Evidence that flavivirus NS1-NS2A cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J Virol. 1995;69(11):7232-43.
27. Wu SF, Liao CL, Lin YL, Yeh CT, Chen LK, Huang YF, et al. Evaluation of protective efficacy and immune mechanisms of using a non-structural protein NS1 in DNA vaccine against dengue 2 virus in mice. Vaccine. 2003;21(25-26):3919-29.
28. Wallis TP, Huang CY, Nimkar SB, Young PR, Gorman JJ. Determination of the disulfide bond arrangement of Dengue virus NS1 protein. J Biol Chem. 2004;279(20):20729-41.
29. Rozen-Gagnon K, Moreland NJ, Ruedl C, Vasudevan SG. Expression and immunoaffinity purification of recombinant dengue virus 2 NS1 protein as a cleavable SUMOstar fusion. Protein Expr Purif. 2012;82(1):20-5.
30. Amorim JH, Porchia BF, Balan A, Cavalcante RC, da Costa SM, de Barcelos Alves AM, et al. Refolded dengue virus type 2 NS1 protein expressed in Escherichia coli preserves structural and immunological properties of the native protein. J Virol Methods. 2010;167(2):186-92.
31. Noisakran S, Dechtawewat T, Rinkaewkan P, Puttikhunt C, Kanjanahaluethai A, Kasinrerk W, et al. Characterization of dengue virus NS1 stably expressed in 293T cell lines. J Virol Methods. 2007;142(1-2):67-80.
32. Masrinoul PP, Diata MO, Pambudi S, Limkittikul K, Ikuta, K, Kurosu T. Highly conserved region 141?168 of the NS1 protein is a new common epitope region of dengue virus. Jpn J Infect Dis. 2011;64(2):109-15.
Received in October, 2012.
Accepted in April, 2013.
Gilda Lemos. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, CP 11600, La Habana, Cuba. E-mail: gilda.lemos@cigb.edu.cu.