Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Estomatología
versión impresa ISSN 0034-7507
Rev Cubana Estomatol vol.49 no.2 Ciudad de La Habana abr.-jun. 2012
ARTÍCULO ORIGINAL
Mucoepidermoid carcinoma of the salivary glands in Brazil: clinicopathological outcomes and a brief review
Carcinoma mucoepidermoide de las glándulas salivares en Brasil: resultados clinicopatológicos y una breve revisión
DDS, PhD. Lucinei Roberto Oliveira,I DDS, MSc. Danilo Figueiredo Soave,II DDS, PhD. João Paulo Oliveira-Costa,II PhD. Miguel Angel Sala Di Matteo,III MD, PhD. Alfredo Ribeiro-SilvaII
I Vale do Rio Verde University (UninCor). Brazil.
II Ribeirao Preto Medical School, University of Sao Paulo. Brazil.
III Faculty of Dentistry of Ribeirao Preto. University of Sao Paulo. Brazil.
ABSTRACT
The biological features and the clinical behavior of the mucoepidermoid carcinoma are varied and not known yet. The aim of present paper was to analyze the potential prognostic factors affecting the survival of patients diagnosed with primary mucoepidermoid carcinoma of head and neck. A retrospective study was conducted in 16 patients treated between 1990 and 2008 in the General Hospital of Riberirao Preto, USP Medicine School, Brazil. The following variables were studied: age, sex, anatomical location, tumor size, clinical stage, histological degree, relapse, metastasis, involved surgical edges ant treatment on the clinical-pathological results. The survival curves were designed using the Kaplan-Meier method and the statistic analysis was made using the log-rank test. The 68.7 % of patients was of male sex, all patients were between 13 and 83 years old. The 75 % of tumors was located in the great salivary glands, the 56.3 % in the parotid glands ones, the mucoepidermoid carcinomas of low degree and of II stage were the 37.5 %. The surgical resection was carried out in all patients. The follow-up period in present study fluctuates between 6 and 217 months. The general rate of 5- y years or 10-years survival was of 85.6 % whereas the rates of disease-free survival were of 81.8 % at 5 years and of 68.2 % at 10 years. The were statistically significant influences of the tumor size (p = 0.05), presence of metastasis (p = 0.04) and of the primary anatomical location (p = 0.04) on the rates of disease-free survival. The results obtained show the significance of the primary anatomical location of the tumor, of its size and of the presence of metastasis in the survival of mucoepidermoid carcinomas.
Key words: salivary glands neoplasms, mucoepidermoid carcinoma, prognosis, disease-free survival.
RESUMEN
Las características biológicas y el comportamiento clínico del carcinoma mucoepidermoide son muy variados y aún poco conocidos. El propósito de este estudio fue analizar los factores pronósticos que puedan afectar la supervivencia de los pacientes con diagnóstico de carcinoma mucoepidermoide primario de cabeza y cuello. Se realizó un estudio retrospectivo de 16 pacientes tratados entre 1990 y 2008 en el Hospital General de Ribeirao Preto, Escuela de Medicina USP, Brasil. Se estudiaron las variables: edad, sexo, localización anatómica, tamaño del tumor, estadio clínico, grado histológico, recidiva, metástasis, bordes quirúrgicos comprometidos y tratamiento, sobre los resultados clínico-patológicos. Las curvas de supervivencia fueron construidas utilizando el método de Kaplan-Meier y el análisis estadístico fue realizado mediante la prueba del log-rank. Se constató 68,7 % de pacientes del sexo masculino, todos los pacientes comprendidos en las edades entre 13 y 83 años. El 75 % de los tumores se localizó en las glándulas salivales mayores, 56,3 % en parótida, los carcinomas mucoepidermoides de bajo grado y estadio II con 37,5 %. La resección quirúrgica fue realizada en todos los pacientes. El período de seguimiento en este estudio varió entre 6 y 217 meses. La tasa general de supervivencia, tanto a los 5 como a los 10 años fue de 85,6 %, mientras que las tasas de supervivencia libre de enfermedad fueron de 81,8 % a los 5 años y de 68,2 % a los 10 años. Se demostró la existencia de influencias estadísticamente significativas del tamaño del tumor (p = 0,05), presencia de metástasis (p = 0,04), y de la localización anatómica primaria (p = 0,04) sobre las tasas de supervivencia libre de enfermedad. Los resultados obtenidos demuestran la importancia de la localización anatómica primaria del tumor, de su tamaño y de la presencia de metástasis, en la supervivencia de los carcinomas mucoepidermoides.
Palabras clave: neoplasias de las glándulas salivales, carcinoma mucoepidermoide, pronóstico, supervivencia libre de enfermedad.
INTRODUCTION
Primary malignant salivary gland tumors represent less than 3 % of head and neck cancers and 10-15 % of all glandular tumors. There is a wide variation in these malignant neoplasms over different geographic areas and ethnic groups. Due to the singularity and diverse histology of these tumors, prognostic factors have been difficult to elucidate.1
The establishment and description of mucoepidermoid carcinoma (MEC) as a distinct salivary gland tumor was originally credited to Stewart et al. (1945).2 Since then, MEC has been described as the most common malignant salivary gland tumor, accounting for close to 10% of all salivary gland neoplasms.3,4
The clinical behavior of MEC is widely variable, but it seems to correlate with tumor stage and grade. MEC generally shows an extremely aggressive pattern for high-grade tumors, whereas its respective low-grade counterpart is often indolent and slow-growing.5,6 However, metastases have been described in some cases of low-grade MEC.7 These discrepancies exist likely because there are several histological graduation systems that have been recommended for MECs,7-10 none of which is universally accepted, which makes the retrospective investigations of clinical outcomes difficult.3 In the same way, although complete resection with free surgical margins is the main treatment for MEC, there is still a lack of consensus on the postoperative use of radio- and/or chemotherapy in some cases.3,4
The mechanisms of pathogenesis and progression of salivary gland tumors are still poorly understood, and studies of prognostic factors that evaluate only one type of salivary gland tumor are scarce for several countries in the world. This study aimed to analyze prognostic factors that may affect survival in patients with a primary diagnosis of head and neck mucoepidermoid carcinomas.
METHODS
A retrospective study was carried out of patients with a primary diagnosis of head and neck MEC treated between 1990 and 2008 at the General Hospital of Ribeirao Preto School of Medicine-USP, Brazil. The medical and surgical records of all cases were reviewed for clinicopathological factors, such as age, gender, primary tumor location, tumor size, clinical stage, histological grade, treatment, compromised surgical margins, tumor recurrence, metastasis, disease-free survival (DFS) and overall survival (OS). The study protocol was performed with prior approval of the local Human Research Ethics Committee (approval number HC/FMRP-USP 10142/2010).
All cases met the criteria proposed by the World Health Organization for the diagnosis of salivary gland MEC.11 Additional inclusion criteria were documented treatment of primary MEC at our institution and a minimum of six months of follow-up information. MECs were staged according to the TNM classification of malignant tumors,12 and minor salivary gland tumors were staged according to their site of origin in a similar fashion to squamous cell carcinomas. Two oral pathologists (LRO and DFS) reviewed all cases to histopathologically classify these tumors according to the protocol published by Brandwein et al. (2001),10 which classifies tumors into low (Grade I), intermediate (Grade II) or high (Grade III) grades. Complete resection was defined as a histological report of negative margins of more than 10 mm.13
Patients' features were summarized through descriptive statistics (mean, range for continuous variables and frequency and percentage for categorical variables). The DFS was calculated as the time interval between the date of first treatment and the date of local disease recurrence or last information for censored observations when the patient was known to be disease-free. The OS was defined as the interval between the beginning of the treatment and the date of death or last information for censored observations. Data concerning survival, recurrence and metastasis were evaluated. The Kaplan-Meier method was used to plot survival curves with the log rank test for analysis of cumulative survival rates. Statistical significance was defined as a 2-tailed P value of = 0.05.
RESULTS
A total of 16 surgically-treated cases of MEC met the inclusion criteria for this survey during the specified period. The clinicopathologic features and results of log rank tests for clinical variables are shown in Tables 1 and 2, respectively.
Table 1. Clinicopathological characteristics of 16 patients diagnosed with mucoepidermoid carcinoma
| Age* | Gender | Anatomic | Size | Treatment | Stage | Histologyc | Recurrence | Metastasis | Disease-free | Overall survival |
| 23 | Male | Retromolar area | 7 | Surg/Rad | IV | intermediate | yes | yes | 80 | 84 |
| 83 | Male | Submandibular | 1,5 | Surg/Rad | III | high | yes | yes | 23 | 70 |
| 76 | Male | Parotid | 3 | Surg | II | high | no | no | 8 | 8 |
| 60 | Male | Parotid | 4 | Surg/Rad | II | high | no | no | 60 | 60 |
| 58 | Male | Submandibular | 3 | Surg | IV | intermediate | yes | yes | 153 | 179 |
| 81 | Male | Parotid | 3 | Surg | III | intermediate | no | yes | 157 | 159 |
| 82 | Male | Parotid | 3 | Surg | II | low | no | no | 16 | 16 |
| 62 | Male | Parotid | 2 | Surg/Rad/Chem | I | low | no | no | 21 | 21 |
| 31 | Male | Palate | 4 | Surg/Rad/Chem | II | intermediate | no | no | 6 | 6 |
| 51 | Parotid | 2,5 | Surg | II | low | no | no | 60 | 60 | |
| 38 | Male | Parotid | 1 | Surg | I | low | no | no | 16 | 16 |
| 64 | Female | Parotid | 2 | Surg | I | intermediate | no | no | 217 | 217 |
| 13 | Female | Palate | 3,5 | Surg | II | low | no | no | 70 | 70 |
| 78 | Female | Parotid | 2 | Surg | I | high | no | no | 95 | 95 |
| 39 | Female | Palate | 1 | Surg | I | low | no | no | 83 | 83 |
| 67 | Female | Sublingual | 3 | Surg | III | high | yes | yes | 22 | 34 |
* Age (years)
Surg = Surgery
Rad = Radiotherapy
Chem = Chemotherapy
Table 2. Results of log test for clinical variables foun in the 16 patients diagnosed mucoepidermoid carcinomas
| Clinical variable | Overall survival after 5 years (%) | P value | Disease-free survival (%) | P value |
| Age (years) | ||||
| ≤ 60 > 60 | 100 72,9 | 0,182 | 100 60 | 0,567 |
| GENDER | ||||
| Male Female | 77,1 100 | 0,201 | 83,3 80 | 0,824 |
| Anatomical site | 0,042 | |||
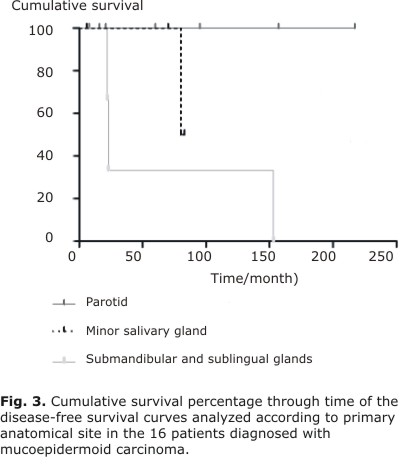
| Parotid Minor glands Submandibular/sublingual | 74,1 100 100 | 0,749 | 100 100 33,3 | |
| Tumor size | ||||
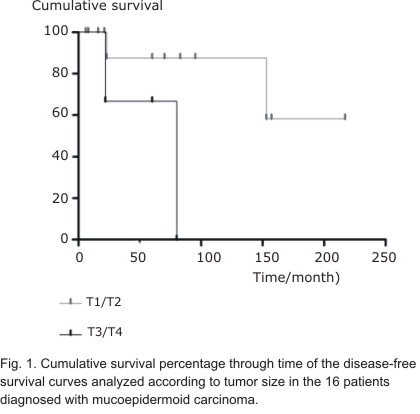
| T1/T2 T3/T4 | 100 81,5 | 0,445 | 87,5 66,7 | 0,053 |
| Clinical stage | ||||
| EI/EII EIII/EIV | 100 77,1 | 0,919 | 100 60 | 0,335 |
| Histological grade | ||||
| Low Intermediate High | 75 100 80 | 0,818 | 100 100 50 | 0,166 |
| Margins | ||||
| Compromised Free | 79,5 100 | 0,805 | 71,4 100 | 0,605 |
| Treatment | ||||
| Surgery Surgery plus adjuvant | 90,9 75 | 0,536 | 87,5 66,7 | 0,504 |
| Recurrence | ||||
| Yes No | 79,5 100 | 0,211 | - | - |
| Metastasis | ||||
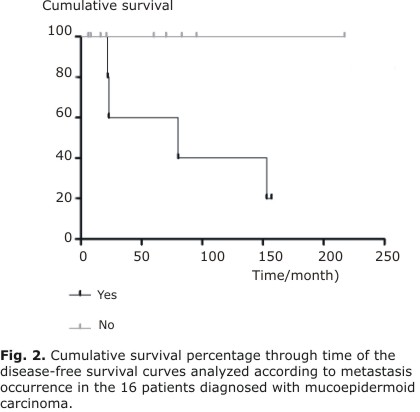
| Yes No | 77,1 100 | 0,931 | 60 100 | 0,043 |
The follow-up period in this study ranged from 6 to 217 months (median 65 months), and the 5- and 10-year OS rates were both 85.6%. The disease-free interval for recurrences and metastases ranged from 22 to 153 months and 22 to 157 months, respectively. Rates of 81.8% and 68.2% were found for DFS over a period of 5 and 10 years, respectively.
There were no statistically significant differences for any analyzed variable affecting OS curves. However, as illustrated in Figures 1 and 2, the DFS curves showed significant differences for tumor size (P = 0.05) and metastasis (P = 0.04), respectively.
Additionally, a significant influence on prognosis was observed in DFS curves depending on the primary anatomical site. Tumors were stratified into three groups: parotid, minor glands and submandibular/sublingual glands. All tumors diagnosed in the submandibular and sublingual glands had recurrences and metastases (Table 1) and, consequently, a worse clinical outcome (P = 0.04) (Figure 3).
DISCUSSION
The MEC is the most common malignant neoplasm observed in the major and minor salivary glands, comprising about one-third of all patients affected by salivary malignancies.14 Diagnosis can occur over a wide age range but occurs predominantly between the third and sixth decades and affects slightly more women than men (3:2). Almost half of these tumors occur in the major salivary glands, appearing predominantly in the parotid (45%).10 However, due to its infrequency and histopathological diversity, the epidemiological distribution pattern of primary MECs observed in one country can differ from others, and there are still few published studies investigating the behavior of these tumors through long follow-up periods. In the present study, we set out to examine MEC behavior and patient outcomes to provide additional information on potential factors that could significantly affect the prognosis of these tumors.
Our results are similar to reported by Triantafillidou et al. (2006)14 who found 16 cases over a 15-year interval. The broad age range observed in our series supports that reported in literature.4,13 Although there are a few studies corroborating our results,13,15 the masculine gender prevalence found differed from commonly reported data that patients diagnosed with MEC are predominantly female.4,16
In agreement with most other studies,4,5,13,15 we found a prevalence of primary tumors diagnosed in the major salivary glands, especially in the parotid gland, followed by intraoral MECs identified in minor salivary glands sited in the hard palate.16,17 In contrast with other investigations,4,13,15 there was an unusual case of a MEC found in the sublingual gland.
As in most other studies,4,6,13 we found a predominance of MECs diagnosed in early clinical stages as T1/T2 and our findings showed a significant influence of tumor size on DFS.4,15 The low rates of recurrence and metastasis found in our study are in agreement with previous reports.4,6 A significant influence of primary anatomical site was observed in DFS curves when there was stratification into three groups because all tumors diagnosed in the submandibular and sublingual glands had recurrences and metastases, which negatively affected the prognosis, this finding confirms previous observations.7,18
Despite several attempts, an established grading system for MEC does not yet exist. The three-level grading system commonly used by pathologists for MEC classification mainly considers the relative proportion of cell types (epidermoid, intermediate and mucinous cells), their respective degrees of atypia and growth patterns (cystic, solid, or infiltrative), together with neural and vascular invasion.7,10 The grading system proposed by Brandwein et al. (2001)10 was adopted in our study because it is objective and easy to use and reproduce,3,4 which will help future standardization. Unlike other studies that used this same grading system,4,13 our results demonstrate a balance in distribution among the three tumor grades subtypes. Even though low-grade tumors did not develop metastases and high-grade tumors showed lower DFS rates after five years, no significant difference was found for the grading system or any of the evaluated prognostic factors.
In contrast to results reported by Nance et al. (2008),4 we did not observe any association in our study between positive surgical margins and decreased DFS. Although MEC has been described as a radioresistant tumor, postoperative radiotherapy has been associated with decreased recurrence in some reports.13 Conversely, in the present investigation, a trend toward better survival was demonstrated in the group who underwent surgical treatment alone, although this finding was not statistically significant. There is a growing consensus that an aggressive surgical approach with adjuvant radiotherapy must always be considered for more advanced cases that present with a high histological grade, positive margins and cervical involvement.3,4,15,19
The clinical progression of MEC is usually slow and, therefore, requires long-term follow-up to establish prognostic factors that could influence clinical outcome. Published works usually lose relevant survival information through time. Although this current investigation was limited by a relatively small sample size, there was a long follow-up period in which we could verify and confirm the influence of some prognostic factors. Further investigation of potential factors that may influence the survival of these patients should be encouraged through longer follow-up periods and larger samples.
Through a long follow-up period in present study we could highlight the relevance of primary anatomical site, tumor size and metastasis as useful prognostic factors that may affect survival in patients with a primary diagnosis of head and neck mucoepidermoid carcinomas. Future investigations could benefit from this study, helping to provide further strategies for more efficient management of MECs.
BIBLIOGRAPHIC REFERENCES
1. Drivas EI, Skoulakis CE, Symvoulakism EK, Bizaki AG, Lachanas VA, Bizakis JG. Pattern of parotid gland tumors on Crete, Greece: a retrospective study of 131 cases. Med Sci Monit. 2007;13(3):CR136-40.
2. Stewart FW, Foote FW Jr, Becker WF. Mucoepidermoid tumors of the salivary glands. Ann Surg. 1945;122:820-44.
3. Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13(6):293-07.
4. Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT, et al. Treatment and survival outcomes based on histological grading in patients with head and neck mucoepidermoid carcinoma. Cancer. 2008;113(8):2082-9.
5. Guzzo M, Andreola S, Sirizzotti G, Cantu G. Mucoepidermoid carcinoma of the salivary glands: clinicopathological review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9(7):688-95.
6. Kokemueller H, Brueggemann N, Swennen G, Eckardt A. Mucoepidermoid carcinoma of the salivary glands-clinical review of 42 cases. Oral Oncol. 2005;41(1):3-10.
7. Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathological analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217-24.
8. Batsakis JG, Luna MA. Histopathological grading of salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol. 1990;99(10 Pt 1):835-8.
9. Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer. 1992;69(8):2021-30.
10. Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathological study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835-45.
11. Seifert G, Sobin LH. Histological classification of salivary gland tumours. En: World Health Organization. International histological classification of tumours. Berlin: Springer-Verlag; 1991.
12. Wittekind C, Greene FL, Hutter RVP, Klimpfinger M, Sobin LH. TNM Atlas: Illustrated Guide to the TNM Classification of Malignant Tumours (UICC). Heidelberg: Springer; 2004.
13. Rapidis AD, Givalos N, Gakiopoulou H, Stavrianos SD, Faratzis G, Lagogiannis GA, et al. Mucoepidermoid carcinoma of the salivary glands. Review of the literature and clinicopathological analysis of 18 patients. Oral Oncol. 2007;43(2):130-6.
14. Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. Mucoepidermoid carcinoma of minor salivary glands: a clinical study of 16 cases and review of the literature. Oral Dis. 2006;12(4):364-70.
15. Ozawa H, Tomita T, Sakamoto K, Tagawa T, Fujii R, Kanzaki S, et al. Mucoepidermoid carcinoma of the head and neck: clinical analysis of 43 patients. Jpn J Clin Oncol. 2008;38(6):414-8.
16. Bernardes VF, Ramos-Jorge ML, Vieira Carmo MA, Cardoso SV, Alves Mesquita R, Ferreira Aguiar MC. Intraoral Mucoepidermoid Carcinoma of Salivary Glands: Lack of Association Among Clinicopathological Features and Immunoexpression of c-erbB-2 in 29 Cases. Int J Morphol. 2008;26(4):1005-11.
17. Lopes MA, da Cruz Perez DE, de Abreu Alves F, de Almeida OP, Kowalski LP. Clinicopathological and immunohistochemical study of intraoral mucoepidermoid carcinoma. Otolaryngol Head Neck Surg. 2006;134(4):622-6.
18. Wahlberg P, Anderson H, Biörklund A, Möller T, Perfekt R. Carcinoma of the parotid and submandibular glands-a study of survival in 2465 patients. Oral Oncol. 2002;38(7):706-13.
19. García-Roco Pérez O. Tumores de glándulas salivales: Su comportamiento en 10 años de trabajo (1993-2002). Rev Cubana Estomatol [online] 2003;40(3). Disponible en: http://www.bvs.sld.cu/revistas/est/vol40_3_03/est01303.htm
Recibido: 17 de junio de 2011.
Aprobado: 15 de noviembre de 2011.
Dr. Alfredo Ribeiro-Silva. Department of Pathology, Ribeirao Preto Medical School, University of Sao Paulo, Avenida Bandeirantes 3900, 14049-900, Ribeirao Preto, Sao Paulo, Brazil. Phone: +55 16 3602 3172 _ Fax +55 16 3633 1068. Correo electrónico: arsilva@fmrp.usp.br