INTRODUCTION
Tooth enamel is the most mineralized structure in the body. It behaves like a dynamic semi-permeable membrane that maintains its structure.1 However, its acellular condition does not allow self-repair following the loss of the mineral portion.2,3)
Research is being carried out to remineralize enamel with calcium phosphates by means of minimally invasive techniques.4,5 For this reason, it is necessary to have statistically significant data on the origin, degree, and location of demineralization, which is challenging and complicated to study in a natural way.
The literature has reported numerous models of chemical demineralization by means of organic acids (lactic and acetic) with exposures between 16 hours and 2 days,6,7 through processes of cycling of demineralization and remineralization to reproduce the oral conditions as described by Cheng et al.8) De Campos et al.9 and Arthur et al.10 have obtained comparable results with an early demineralization model using biological models.
These models generate artificial incipient lesions but most of them are slow, complex and costly processes, with limited reproducibility and high variability. Sabel11 used an in vitro method of demineralization of deciduous teeth enamel by the use of lactic acid at pH= 5.3 applied at 37 °C for 30 days. Initial experiments trying to strictly stick to Sabel´s method proved to be unreliable and finally impossible. This simple experimental design seemingly easy to reproduce because of the chemicals and conditions involved lacks reproducibility because there is quite a bit of essential information missing. There are three kinds of lactic acid and this is not pin-pointed in Sabel´s paper where a reliable concentration and volume are also missing. There are many types of carboxymethylcellulose (CMC) that differ in molecular weight, molecular substitution grade, viscosity (high, medium or low), pH and sodium content, this data is not even mentioned in Sabel´s paper. These undefined variables lead to non-reproducible pH values and unspecified viscosities. The use of different types of CMC containing variable amounts of sodium render different pH values. This is why the present study´s objective was to propose this modified simple low-cost and quick method avoiding the use of CMC, and completely stating the type, the grade and concentration of lactic acid.
METHODS
Sample collection and management
The present study proceeds through a completely randomized with dual replicas factorial statistical experimental design.
With the approval from CIE-0202-12 of the Ethics Committee of the Faculty of Dentistry, Universidad Nacional de Colombia, and after the written informed consent of the donors and the delivery of informative primer, 12 third human molars from healthy donors requiring extraction were collected. The samples did not include any teeth extracted with associated diagnosis of periodontal disease, pulpal pathology, caries or fractures. Teeth with moderate or severe pre-eruptive defects in the enamel, amalgam, adhesive restorations, rehabilitation and internal or external whitening were excluded. The specimens were deposited in polyethylene of high density HDPE containers with an air tight seal and stored in 10 mL of chloramine T 0,5 %. The protocols for disinfection and storage of the Biobanco de Dientes Universidad Nacional de Colombia were followed.12)
Distribution and treatment of the sample
The sample (n= 12) was distributed into two groups: Group 1 lactic acid at pH 2,4 and Group 2 (lactic acid at pH 5,4. In addition, each group was further subdivided (n= 2) to evaluate the effects of three exposure times in days (t1= 7 t2= 15, t3= 30). The lapse selection was based on preliminary experiments to detect a measurable change in mineralization, it was decided then that the shortest time should be around one week and t2 and t3 were arbitrarily selected as two and four times one week. Since a quick method was the purpose, longer times were not considered. The specimens were immersed in lactic acid solutions at 37 °C in a thermostat. After demineralization, the specimens were washed with distilled water, labeled and stored in isotonic synthetic saliva at 4 °C. The sample size was 12 specimens, to which a completely randomized factorial experiments design was carried out with two replicates, the factors being pH (two levels: pH1= 2.4 and pH2= 5.4) and the time in days (three levels: t1= 7, t2= 15 and t3= 30). The power of the test was computed following the methodology proposed by Cohen, which rendered 95.3 %. Values exceeding 80 % are deemed high, however due to the small number of replicates per subgroup (n= 2), it is estimated that the power falls to approximately 36 %. That is the reason for a completely randomized design of factorial experiments with six replicates, taking into account the pH factor (at two levels: pH1= 2.4 and pH2= 5.4), where it was found that the power of the test corresponds to 99.1 %.
Surface isolation method
The experimental area of vestibular face of each specimen was isolated with a 4 mm x 3 mm window of PVC tape. The remainder of the coronal and radicular surface was covered with an acid-fast nail varnish and overlaid with a polyvinyl film to ensure that only the specified vestibular surface was exposed to the treatment.
Preparation of the demineralization solution
Two demineralization solutions were prepared with racemic lactic acid 90% concentration USP (United State Pharmacopoeia) grade, without using carboxymethylcellulose. Solution 1 had a pH of 2.4 (2.8298 g of lactic acid with ISO grade I demineralized water to 250 mL) determined using a pH meter and a glass electrode with a three point buffer calibration. Solution 2 had a pH of 5.4 (10 mL of the above solution with 250 mL demineralized water). pH values were selected as stated in literature as critical pH values for onset of dental enamel demineralization.4
Criteria for evaluation of demineralization
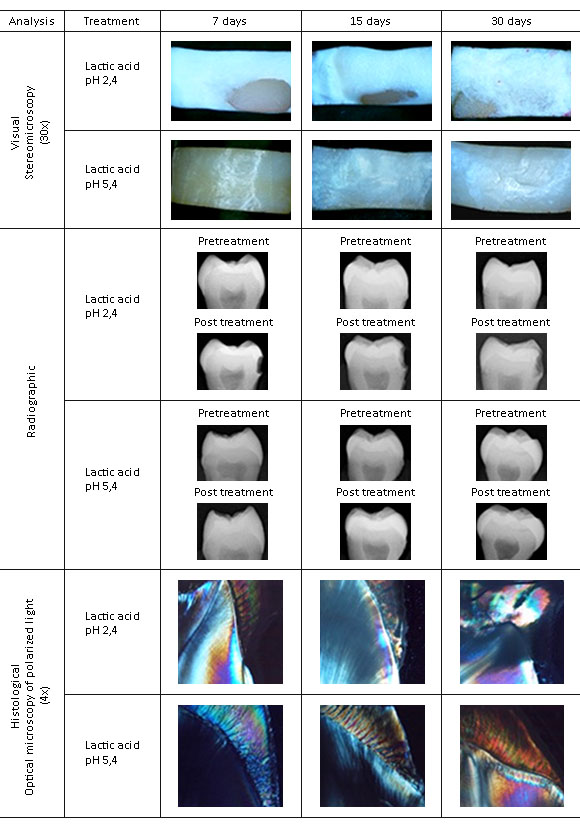
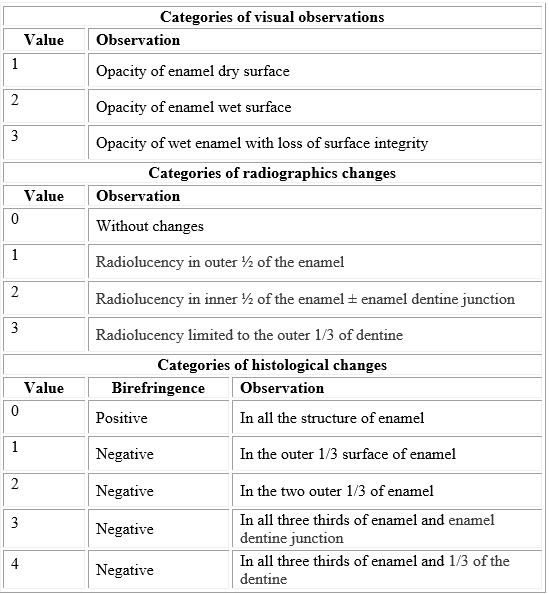
After demineralization, visual, radiographic and histological changes were analyzed and organized into categories for statistical analysis (annex).
Visual Analysis
Images of the vestibular surface were obtained for pre-and post- acid treatment, using the Nikon SMZ800 stereomicroscope with 20x and 30x objectives, to identify changes in the tooth surface. The results were categorized based on the visual diagnosis of caries as described in the International Caries Detection and Assessment System (ICDAS) (annex).
Radiographic analysis
Pre- and post-treatment images of the dental enamel were obtained using a periapical X-ray (GenDex Gx-770) with constant conditions of capture (voltage of 70 kVp, current of 7 mA/s, exposition times of 5/60s and 10/60s, with the largest axis of the specimen perpendicular to the bundle of X-rays). The images were also analyzed pre-and post-treatment by a digital analysis software (Dental Imaging Software - Carestream dental; Kodak®). Two types of tests were performed:
-Direct X-ray analysis: The best X-ray images were chosen, and values were assigned according to the structural changes resulting from the demineralization (annex).
-Software-based analysis of digital images: Because radiographic changes in some specimens could not be seen via the direct observation of the radiographic images, we proceeded to quantify the gray scale using a software of digital image analysis, which ranged from 0 to 255, with 0 being the black color value and 255 the white color value. This quantification was completed on a segment of the image between the outer enamel surface and the dentinal projection of the horn pulp of each specimen. To facilitate the observation of changes in the scale of gray between both captures, we used the color scale tool of the software, with a calculation of the average value.
Histological analysis
The samples were then prepared for polarized light microscopy. To obtain sections of the samples with a 0.5 mm average thickness, coronal cuts were conducted in a sagittal direction with a low speed (Isomet Low Speed Saw) precision cutting machine; Buehler) with a diamond disc (size 4).Subsequently, the sections were slimmed with carbide discs in an electrical microengine (Mighty M-3 Marathon; Saeyang Microtech) and with sandpaper with a grain number 1200 (Mesh scale for particle size distribution corresponding to approximate 12 μm). The sections were mounted on microscope slides with a resin for histological use (Citoresin-60; BER Escorcia & CIA ltda) and were observed in the polarization microscope (Olympus BX51) under 4 x, 10 x and 20 x objectives with a standard polarizer angle of 90°, and images were captured using the microphotographic camera (Olympus DP12 with a CCD detector of 3.2 megapixels). Finally, values were assigned according to the location of the demineralization expressed as absence of birefringence (annex).
Statistical analysis
The handling of the data was performed in three parts:
1. Differences (ΔDOG) between the final and initial radiographic density of gray (DOG) was determined. A test of the equality of means was also completed, (H0 ΔDOG= 0) for the different periods of time. The alternative hypothesis was that the mean of DOG in the untreated specimens was higher than the mean after the treatment.
2. Index of integration of the answers: After a quantitative answer was produced (from the scale measurement of gray) and the rest of the answers of ordinal type were obtained (visual, radiographic and histological), and knowing that each of them generates important information on the analysis, an index was constructed to simultaneously assemble in a single variable the integration of the four observations methods on the final result of the treatment. For the construction of the index, multivariate statistical methods were used. In particular, multiple factor analysis (AFM) allowing for the integration of quantitative and qualitative variables. This method consisted of creating an ideal quantification of the ordinal variables and assigning numerical values to them and the categories (annex) in such a way that the restrictions of order would be respected.13
3. ANOVA: several tests were carried out to validate the assumption of normality (Shapiro-Wilk, Anderson-Darling, Cramer-von Mises, Pearsochi-square), and none of them rejected the null hypothesis that the errors come from a normally distributed population. To verify the homoscedasticity, we conducted the Bartlett test, leading to the conclusion that the studied samples had the same variance.
4. The analyses concluded with the analysis of the variances.
RESULTS
Qualitative results
The visual, radiographic and histological effects are shown in figure, and the values according to the categories for statistical management are provided in table 1. In general, demineralization was observed with a high structural involvement of the dental enamel in the G1, extending to the dentinal tissue when it was exposed during the greatest time of application to the solution. In the G2, there was an incipient alteration in the detectable mineral content at the visual and radiographic levels, although it was not obvious by direct imaging and instead could only be appreciated from differences in the DOG. The findings were confirmed at the histological level.
Table 1 Results of categorizing qualitative observations
| Specimen | Visual | Radiographic | Histological | Differences in the shades of gray |
|---|---|---|---|---|
| T1pH1t1 | 3 | 1 | 3 | 105 |
| T2pH1t1 | 3 | 2 | 3 | 142 |
| T3pH1t2 | 3 | 1 | 3 | 129 |
| T3pH1t2 | 3 | 2 | 3 | 108 |
| T5pH1t3 | 3 | 3 | 4 | 130 |
| T5pH1t3 | 3 | 3 | 4 | 108 |
| T7pH2t1 | 2 | 0 | 1 | 49 |
| T7pH2t1 | 1 | 0 | 1 | 53 |
| T9pH2t2 | 1 | 0 | 1 | 77 |
| T9pH2t2 | 2 | 0 | 2 | 80 |
| T11pH2t3 | 2 | 0 | 2 | 45 |
| T11pH2t3 | 1 | 0 | 2 | 55 |
T#= Tooth (1,2,3,...,12); pH= pH of the acid solution (pH1= 2,4; pH2= 5,4); t= exposure time (t1=7 days, t2= 15 days, t3= 30 days).
Grayscale density differences
The comparison of the values of DOG with a statistic t= 8,9856 and 11 degrees of freedom showed that, the average value of the teeth without treatment was greater than the average value of the treated teeth. The mean DOGs at the beginning and at the end were 199,2 and 109,1, respectively (p= 0,005).
Index of the integration of answers
The index of integration of the demineralization levels of the dental enamel of the specimens is shown in table 2. This index gives negative and positive values because the multiple factor analysis includes both in this scale, reflecting the order in which a few specimens are more affected than others. The interpretation of the results used a sample with treatment at pH1 and time 3 (30 days) as the state with 100 % of the treatment effect (%TE). From this assumption, an affect grade was assigned to each specimen. This test allowed us to determine that the greatest damage occurred at a pH of 1 (2.4) and that the pH of the solution influences the results more than the time does.
Table 2 Results of Index of integration of the four observations methods
| Index value | 1,32 | 1,12 | 1,1 | 0,8 | 0,76 | 0,75 | 0,69 | -0,69 | -1,05 | -1,06 | -1,14 | -1,23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 |
| Exposure time | 3 | 1 | 2 | 2 | 1 | 3 | 3 | 1 | 1 | 3 | 2 | 1 |
| Index as percent treatment effect (%TE) | 100 | 93 | 92 | 82 | 80 | 80 | 29 | 29 | 16 | 16 | 13 | 10 |
Finally, the analysis of variance illustrated in table 3 showed that there are significant differences between the effects caused by the pH of the solutions, and these effects were more pronounced than the effect of the time (p= 0,505).
DISCUSSION
The methods of dental demineralization in vitro are useful in mineralization studies because they can reproduce the loss of mineral in the dental structure, such as those caused at the oral environment by large imbalances in the pH14 either erosive,15) bacterial16 or certain dental procedures.17
The limitations for experimental reproducibility imposed in the method described by Sabel et al.11 because of the lack of materials specifications (CMC molecular weight, substitution grade, viscosity range, lactic acid concentration and type), led to the quick method here proposed by providing enough chemical detail to allow for easy reproduction. There is still a further difference between the two studies since in this documented evaluation third human molars were employed while Sabel used primary molars. The findings were consistent with the results found in some specimens reported by Sabel after using a demineralization solution at a pH of 5.3 for 30 days.11)
The method evaluated in the present study obtained results in a simple, economical way and in a short time compared to that reported in the literature. Visually, the lactic acid solution at the pH of 5.4 produced a change in the opacity and a loss of brightness on the surface of the enamel without compromising the surface integrity, as detected during the 7 days of treatment and reported in several studies of the chemical methods of demineralization with solutions of lactic acid or acetic acid at a pH of 4.8.18,19,20 However, opacity was accompanied by a loss of surface integrity represented by the presence of a soft layer of enamel, which relates to erosive lesions. This was previously reported in the work of Lonta et al, who simulated erosive lesions in dentin with a solution of citric acid at a pH of 2.4,21 and Nakata et al., who used lactic acid at a pH of 5.0 for longer periods for up to 7 weeks.22
The literature reports the usefulness of the radiographic identification of advanced demineralization.23 Using the gray density tool of the imaging software, an early enamel demineralization was identified in a process comparable to more costly and complex methods, such as optical coherence tomography (OCT),18,24,25) transverse microradiography (TMR)19 and quantitative fluorescence induced by light (QLF)18) This finding thus extends the possibilities for the use of digital periapical radiography associated with image analysis software, as an economic tool with easy access for the evaluation of changes in the mineralization in dental enamel.
Histologically, the literature reports that QLF can determine changes in the fluorescence following the loss of minerals.26 Polarization microscopy asserted the structural changes in the birefringent subsurface resulting from demineralization27 at a pH of 5.4 and the underlying dentine produced at a pH of 2.4, as reported by Miresmaeili et al.28 and Polishing et al.29
Synthetic indices devised for integrating all the qualitative and quantitative statisticals here obtained, combined with the visual, radiographical and histological analysis demonstrated that lactic acid with a pH of 5.4 applied to the dental enamel for 7 days was effective in creating a superficial demineralization of the dental enamel. The results were similar to injuries of early demineralization achieved by more complex, lengthy, and expensive methodologies, like those of biological type10 or chemical cycling type.30
It is necessary to mention that we produced erosive grades 1 and 2 according to the literature15 with the solution of pH 2.4. This finding provides valuable information that can support and contribute to the development of research on that specific topic.
In conclusion, the method of in-vitro demineralization with lactic acid at pH 5.4 for 7 days is effective in creating an incipient demineralization similar to that observed in an early clinical lesion of the dental enamel. The lactic acid solution at pH 2.4 produced injuries in the tooth enamel that were more consistent with significant dental erosion even at the shortest time. Tools and equipment such as polarization microscopy, stereomicroscopy, periapical digital radiography fitted with image analysis software were found to be useful for the qualitative analysis of changes in the mineral content of tooth enamel.