INTRODUCTION
The alveolar process is a tooth-dependent structure, and its development is strictly connected with tooth eruption. Several changes could occur after tooth extraction, including a loss in height and width of the alveolar bone. Implant therapy can be considered successful only when functional and esthetic requirements have been accomplished. Therefore, both adequate alveolar bone volume and favorable alveolar ridge architecture are important considerations to obtain a positive functional and esthetic rehabilitation. Socket preservation procedures have been performed to preserve hard and soft tissue volume, which can be partially lost after tooth removal.1
An ideal situation would be that the extraction site heals with a bone formation that completely preserved or recreated the original dimensions of the ridge when the tooth was present. Unfortunately, bone resorption is common after tooth extraction, which thus creates the need to intervene with a method to provide ideal bone for implant placement and reconstruction with an esthetic and functional restoration.2) Successful management of the extraction socket can be challenging, particularly in the esthetic zone.3) Different techniques appear to reconstruct lost tissue with enough bone volume for the placement of an osseo-integrated dental implant. The materials chosen to graft the extraction socket should include the following qualities:
1. The material should maintain space for the bone to repopulate the graft and thus recreate the bone volume similar to the original.
2. The bone formed should have the density to allow for stabile placement of the implant; thus, the material placed should have exciting osteoconductive features to enhance bone formation.
3. The material should be relatively inexpensive and readily available.
There are no cases reported in the literature with loss of the palatal plate, probably due to its complex regenerative process. This case report aimed to describe prosthodontic treatment in an extraction socket with advanced palatal bone resorption, through the use of an implant-supported single fixed prosthesis.
CLINICAL REPORT
39-year-old male patient, without systemic disease, and completely dentate with no occlusal parafunction. The radiographic exam showed the presence of advanced palatal bone resorption (almost total loss of the palatal plate in all its extension (7 mm) secondary to a root fracture of the maxillary left lateral incisor and a large osteolytic area on the palatal aspect of the root (Fig.1, A). The patient’s main complaint was aesthetic.
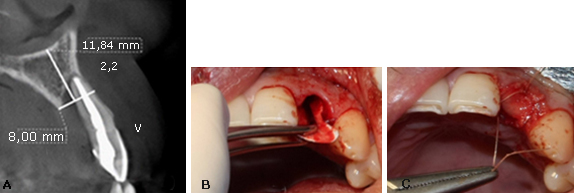
Fig. 1 A: Aspect of the root. B: Resorbable collagen membrane adapted to the bone defect. C: Retaining the bone allograft and closing the socket reparation.
In a first surgical phase, the patient was anesthetized with two tubes of mepivacaine 3 % (Scandicaine, Septodont). The lateral incisor was extracted using an atraumatic periotome technique. Then, a resorbable collagen membrane (BioMend Extend, Zimmer Biomet Dental) (15 mm x 20 mm) was adapted to the bone defect and placed in a position to recreate the palatal plate (Fig. 1, B). After this, particulate cortical bone allograft (Puros Cortical Allograft Particulate, Zimmer Biomet Dental) (1 cc) was compacted into the site to fill the space that was previously occupied by the root of the tooth. The membrane was retained in position with three horizontal mattress sutures (Polysorb 4-0,Covidien) to the buccal free gingiva, retaining the bone allograft and closing the socket reparation (Fig. 1, C). Finally, the temporary restoration was performed using the extracted natural tooth, which was adhesively bonded to the adjacent teeth (Fig. 2, A).
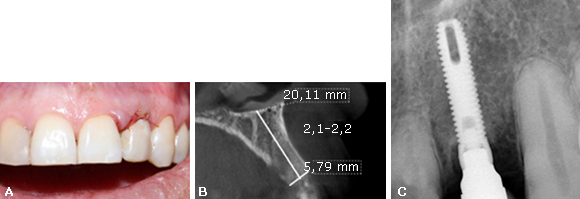
Fig. 2 A: Temporary restoration using the extracted natural tooth. B: Four months after grafting computed tomography (CT) scan showed an adequate height and width of the bone. C: Implant placed in the second surgical phase.
Four months after grafting the extraction site in preparation for dental implant placement, conventional radiology and computed tomography showed an adequate height and width of the bone (Fig. 2, B). In a second surgical phase, one implant was placed (Screw-Vent, Zimmer Biomet Dental) (3,3 x 16 mm) with an average surgical torque of 25 Ncm (Fig. 2, C). Then, an implant fixture mount was cut slightly above the tissue level and established the emergence profile by simply adding composite (flowable), until the desired contours were achieved. It is important to note that this step should be done extra-orally to prevent the contact of any debris with the surgical site. Subsequently, this custom healing cap was attached to the implant.
Six months after the implant placement, osseointegration was clinically confirmed and a provisional crown screwed on the implant was performed. After several stages, the final restoration with a zirconium dioxide abutment and a full ceramic crown was obtained and cemented (Fig. 3, A).

Fig. 3 A: Six months after the implant placement. B and C: Radiographic and clinical follow-up after two years.
Radiographic and clinical follow-up after two years showed a favorable evolution of the implant. No mechanical or biological complications were seen throughout this observation period. The buccal and palatal gingival margin are in a correct position and there was a vertical bone gain of approximately 2 mm. (Fig. 3, B and C).
DISCUSSION
Papaspyridakos et al.2 describe that the aesthetic rehabilitation of the anterior zone in patients who lose teeth prematurely continues to represent a huge and difficult challenge for implantology. This case report describes the resolution of an implant-supported rehabilitation with an unusual defect and its follow-up after to 2 years.
In this case report, an anterior straight forceps and a periotome were used to remove the tooth and prevent trauma of the alveolar bone. This shows that minimally invasive extraction and grafting techniques satisfied the critical requirements needed for socket repair, as Barone et al.1 research pointed.
In this case, a resorbable collagen membrane and bone allograft was adapted to the bone defect to recreate the palatal plate. Guided bone regeneration has proved to be a suitable and somehow predictable technique for promoting bone regeneration, as the case report of Chaple and Baganet,4 where they repair a bone fenestration with this procedure in a vestibular defect site. The membrane Biomend enhanced the early osteoblast attachment reported in a study. Extraction sites in the aesthetic zone present an obvious restorative challenge. This single-stage approach preserves site morphology by protecting and supporting existing hard and soft tissues.
Clinical success appears to be attributable to several important features of the technique.5
In this case report, our results showed that the regeneration of the palatal plate was possible using particulate cortical bone allograft and a resorbable collagen membrane. Sterio et al.6 has been reported that human mineralized bone in particulate form, preserve the site’s bone bulk and volume in preparation for dental implants placement. Healing of defects packed with particles larger than 300 mum was slower than with 300-90 mum grafts. Fast healing of bone defects packed with particulate bone allografts in the range of 300 to 90 mum, indicates that such allografts can be used effectively in the filling of bone defects.7 In this case, the particle size matches with the bone allograft recommendation. The advantages of using an allograft includes that the graft material is available without the need of a second surgical harvest site, and that the material is osteoconductive. A human case report conducted by Elian et al.,3 reported that five months after grafting with mineralized human bone, osteocyte nuclei within lacunae in an osteoid matrix were found. This matrix was appositionally deposited against non-vital graft bone.
The combination of mineralized, freeze-dried, cortical allograft with a nonresorbable porous membrane has resulted in successful bone formation over an extraction site. When using a nonresorbable porous membrane, primary closure of the extraction site is mandatory. Current technique of Attia et al.5 advocates the use of a fast-resorbing material to retain the graft and promote epithelialization over the graft. The graft can be covered with a collagen material that resorbs in less than seven days.
Attia et al.,5 also set that there is insufficient reliable evidence to provide recommendations on which is the ideal flap design, the best soft tissue augmentation technique, the ideal technique to increase the width of keratinised/attached mucosa, and which are the best incision/suture techniques/materials.
Guide bone regeneration in a lateral maxillary incisor is difficult; its histological and anatomical characteristics complicate the clinical protocol. A recent study of Papaspyridakos et al.,2 conducted with cone beam computed tomography technology, reported that spongious bone thickness in the maxillary lateral incisors is slightly smaller than in the central incisors and canine. In both, palatal and vestibular aspects, is important to consider anatomical situation for the regenerative process.
Despite the medium-term success of the treatment reported in this clinical case, is important to emphasize that an adequate training and competences of the operator are necessary to achieve a successful outcome in similar cases. The process of guided bone regeneration is critical to subsequently achieve an optimal installation of the dental implant.
In the clinical follow-up performed after 2 years the rehabilitation is aesthetically pleasing, the buccal and palatal gingival margin are in a correct position and the interproximal papillae is in class I of Miller. According to the computerized axial tomography, in similar non-standardized cuts, there was a vertical bone gain of approximately 2 mm. Rath et al.8 describe that this vertical gain is important to maintain the stability of the implant in the long term.
This case was performed in the context of a postgraduate program at local University, where patients are assured of institutional follow-up at a lower cost than in private practice. Follow-up and maintenance are also critical in the success of clinical situations similar to those reported.
The regeneration of the palatal plate was possible through the use of particulate cortical bone allograft and a resorbable collagen membrane adapted to the bone defect and placed in a position to recreate the palatal plate. This allowed the installation of an implant 4 months later the procedure. This technique allowed esthetics and functional results using a single fixed prosthesis.














