Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión On-line ISSN 1561-2988
Rev Cubana Farm v.43 n.2 Ciudad de la Habana Mayo-ago. 2009
PRODUCTOS NATURALES
Release of theophylline from hydrophilic matrices containing sodium alginate from seasonal Sargassum cymosum (Phaeophyta)
Liberación de la teofilina a partir de matrices hidrófilas que contienen algfinato de sodio procedentes de Sargassum cymosum (Phaeofphyta)
Itamar Francisco AndreazzaI; Martha Maria MaasII; Marcos Luiz PessattiIII; Tania Mari Bellé BresolinIV
IDoctor en Fármacos y Medicamentos. Profesor Curso Farmacia. Universidade Federal do Paraná (UFPR). Brasil.
IILicenciada en Ciencias Farmacéuticas. Universidade do Vale do Itajaí (UNIVALI). Brasil.
IIIDoctor en Bioquímica. Profesor Curso Biotecnologia-UNIVALI. Brasil.
IVDoctora en Bioquímica. Profesora Curso Farmacia-UNIVALI. Brasil.
ABSTRACT
The sodium alginate extracted from seasonal Sargassum cymosum samples (autumn, winter, spring and summer) in Paciência Beach (Penha- SC-Brazil) showed a viscosity of 18.3, 33.5, 62.6 and 34.2 mPa·s, respectively. Theophylline tablets(60 mg) containing 35 % of sodium alginate samples were analyzed by dissolution profile in a dissolution device at 100 rev×min-1 and 37 °C, using water as dissolution medium, during 6 h. Tablets with the lesser viscous sodium alginate sample (autumn) showed a complete disintegration and drug release after 1 h. Despite all others tablets have exhibited the same drug release mechanism, by diffusion, the winter sodium alginate tablets released > 90 % of theophylline after 3 h, while spring and summer sodium alginate tablets showed similar dissolution profile with a release > 90 % of drug after 6 h. In general, the less viscous polymers showed a faster drug release, but probably other characteristics beside viscosity have played role in this process.
Key words: Sodium alginate, hydrophilic matrix, theophylline, drug release.
RESUMEN
El alginato de sodio extraído de muestras estacionales de Sargassum cymosum (otoño, invierno, primavera y verano) en la Playa de la Paciencia (Penha-SC-Brasil) mostró viscosidad de 18,3, 33,5, 62,6 y 34,2 mPa·s, respectivamente. Tabletas de teofilina (60 mg) que contenían 35 % de muestras de alginato de sodio fueron analizadas a través del perfil de disolución, en un disolutor a 100 rev×min-1 y 37 ºC, utilizando agua como medio de disolución, durante 6 h. Las tabletas con la muestra de alginato de sodio menos viscosa (otoño) mostraron completa desintegración y liberación del fármaco después de 1 h. A pesar de que todas las demás tabletas mostraron el mismo mecanismo de liberación del fármaco, por difusión, las tabletas con la muestra de alginato de sodio de invierno liberó > 90 % de la teofilina después de 3 h, mientras las tabletas de alginato de sodio de primavera y verano presentaron un perfil de disolución semejante con liberación > 90 % de fármaco después de 6 h. En general, los polímeros menos viscosos proporcionaron liberación más rápida del fármaco, pero otras características, además viscosidad probablemente desempeñaron alguna función en este proceso.
Palabras clave: Alginato de sodio, matriz hidrofílica, teofilina, liberación de fármacos.
INTRODUCTION
In Brazil, the economical potential of macro algae is not exploited, despite the large coastal extension and the algae variety. It was carried out, in Santa Catarina State (South of Brazil), an evaluation of sustainable exploitation of Sargassum sp. localized in Piçarras Beach and was observed the biomass seasonal variation and the influence of the temperature and region in algae grow,1 and the influence of the temperature and season in algae growing.1
The alginate is the most skeletal component of all brown seaweeds (phylum Phaeophyta) and it is a linear co-polymer of D-mannuronic (M) acid and L-guluronic (G) acid, arranged in a block-wise fashion along the polymer chain. The ratio of M to G and proportions of MM, GG or MG blocks varies from species to species, the part of the plant, and the season of collection.2 From commercial point of view, the most important property of alginate is their ability to form viscous solutions in water and is therefore of considerable practical importance to characterize an alginate sample.3
Among industrial application of these biopolymers there is the pharmaceutical use as hydrophilic matrix, which have been used extensively to provide controlled release oral drug delivery. A number of natural and modified polysaccharides, such as xanthan, galactomannans, karaya gum, alginates and carrageenan have been shown to be controlled drug release at controlled and perhaps time-independent rates.4 Efentakis and Koutlis5 studied the release of furosemide (a rather poorly soluble drug) from hard gelatin capsules (single-unit) and minitablets (multiple-units) filled in hard gelatin capsules, containing commercial sodium alginate of different viscosity grades. These authors showed that the lower viscosity formulations exhibited greater erosion and drug release in comparison to the higher sustained drug release and lower erosion of high viscosity formulations, with a rather aero-order release mechanism, attributed to swelling and erosion/dissolution process.
The aim of this study was to evaluate seasonal Brazilian sodium alginate (NaA) samples, with different physical characteristics for sustained release purposes as matrix tablet of theophylline, a soluble drug.
METHODS
Materials
Theophylline (97-102 % assay) was purchased from All Chemistry (China, batch S991209). Lactose monohydrate was purchased from Gerbrás (Germany, batch 1073) both being of Pharmacopoeia quality (USP, 2003). All algal samples were collected in Paciência Beach (Penha, SC, Brazil) in autumn, winter, spring and summer, and brought fresh to the laboratory. After the necessary separation of other contaminant seaweeds the samples were washed in potable water and dried at 60 °C, in a forced air oven, for 48h. The algae were milled in a hammer mill (Marconiâ). The particle size analysis was carried out on a vibratory sieve shaker (Bertel, Brasil) using 150, 180, 250, 355 and 425 µm sieves. After shaking for 15 min, the weight of retained samples in each sieve was measured. From plots of powder weight (%) versus size (µm) typical parameters from a particle size distribution were determined: mean particle diameter and standard deviation (sd). The moisture loss was determined in triplicate using an infrared moisture analyzer (model LJ16, Mettler Toledo) until constant weight and the dried yield was calculated in relation to fresh algal material.
Extraction and Analysis of Sodium alginate
The sodium form of alginate was obtained by an adaptation of Hernandéz-Carmona et al.6,7 Powder seaweeds (50 g) were boiled with 500 mL of water for 10 min, for enzymatic inactivation and rapidly cooled with 500 mL of freeze water to 10 °C and filtered by nylon cloth (20 µm of pore size). The residue was stirred with 330 mL of 0.1 M HCl (pH 4.0) for 15 min in order to remove laminaran and fucans3 in the acid extract. The remained alginic acid was convert to its soluble Na salt by treatment with 10 % aq. Na2CO3 in water (1.3 L), adjusting it to pH 10 with 10 %, during 3 h, at 80 °C, under stirring. The suspension was filtrated and the filtrate was cooled to 10 °C and then submitted to a treatment to whiten by the addition of NaOCl 10 %. After homogenization the sodium alginate was precipitated with ethanol (final concentration of 60 % v/v). The precipitate was then submitted to successive washings with water/ethanol from 30/70 to 0/100 to a progressive dehydration of polymer and finally dried under vacuum at 50 °C for 3 h.
The 1 % solutions of NaA seasonal samples in 0.1 M NaCl were analyzed by rotational viscosimeter (Haake, VT 550) with a SV-DIN sensor, at 25 °C, attached to a thermocontroler (DC30, Haake). The viscosity curve was determined with the software Rheowin and the viscosity values (n= 3) at the Newtonian plateau (80 s-1) were taken to compare the seasonal samples.
Preparation and Analysis of tablets
The sodium alginate seasonal samples and the lactose were maintained in a desiccator for 12 h and the former was previously compacted in a rotative tabletting machine (Lawes, 2000 10 PSC) due to its elastic and fibrous aspect. The resulting NaA tablets were triturated with the lactose and theophylline in a mixer. The granules resulting were sifted (850 mm) and mixed in a mortar. Matrix tablets (200 mg) containing 30 % of drug, 35 % of lactose and 35 % of different seasonal NaA samples were compressed in the above rotative tabletting machine, using at a speed of 5 r×min-1 and flat-faced punches of 8 mm diameter. The hardness and friability of tablets (n= 10) were measured in a Hardness Tester (Erweka, TBH 20) and in a friabilometer (Erweka, TA 20), respectively. The drug content uniformity of batches (10 units tablets) was analysed in a spectrophotometer (Shimadzu, UVPC 1601), in a 1 cm quartz cell, at 272 nm.8 The absorbance was read using the water as blank. It was made a control batch, without theophylline (50 % of lactose and 50 % of sodium alginate) for corrected the absorbance of gum during the dissolution test.
In vitro dissolution method
In vitro dissolution was carried out with the rotating paddle method (apparatus 2 of USP, 28, Erweka DT80 apparatus) at 100 r×min-1. The medium used was water (900 mL), maintained at 37 ± 0,5 °C. Sampling was performed over 8 h at predetermined time intervals. The amount of theophylline dissolved in each sample was measured by UV detection at 272 nm (Shimadzu, UVPC160), after filtration and dilution. The percentage of drug released was plotted versus time. The results of physical and chemical properties among the different batches were evaluated by ANOVA followed by "a posteriori" test (Ryan-Einot-Gabriel-Welsch Multiple Range). Software Excel 6.0 was used, and the statistical significance was considered at the 0.01 level.
RESULTS
The S. cymosum samples collected in different seasons were washed, dried and milled. The mean size of seaweed powder were: 283.0, 301.7, 268.4 and 290.4 mm for autumn, winter, spring and summer, respectively. The powder may be classified as coarse powder (USP, 2003) with a unimodal distribution.9
The NaA obtained from algal seasonal samples treated with NaOCl 0,8 % (w/v) resulting in a brownish powder with fiber aspect and with a yield of 13 % (w/w). The analysis of characteristics of seasonal NaA samples is show in table 1.
The loss on drying was about 15 % and the NaA yield was quite different for the season, with a higher yield in winter. The viscosity of the samples was spring > summer = winter > autumn. The viscosity curves of NaA samples showed a pseudoplastic behaviour (date not show).
The matrix tablets were elaborate with these sodium alginate samples and the characteristics of tablet batches are show in table 2.
All matrix tablets showed a hardness > 40 N and are approved for uniformity of content, within 85-115 % of drug content and CV < 6,0 %.8
In vitro dissolution
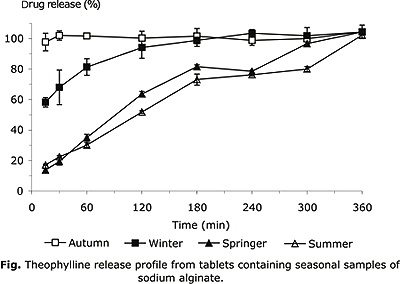
The release profiles of theophylline from NaA matrix tablets are presented in figure. During the drug release studies, all the formulations were observed for physical integrity at different time.
Theophylline was released in a controlled manner over 6 h from the winter, spring and summer batches, but for the autumn matrix tablet the release was immediate, dissolving completely after 1 h. The later contains the less viscous polymer. The other formulations remained as gelatinous mass. The winter batch released 100 % of drug after 3 h while the spring and summer matrix tablets presented a similar profile, releasing around 100% of drug only at the end of experiment.
Although the more viscous polymer (from spring) showed high drug retention, other polymer, as summer sample, with a half of viscosity value, showed a similar behavior that suggest the influence of other characteristics on the biopharmaceutical behavior of the NaA hydrophilic matrices.
In order to describe the dissolution profiles, mathematical models of zero order, first order and Higuchi model (Eq.01) were applied to data.
Q(t)= KH1/2 Eq. 01
where Q is the amount of drug released at time t and KH is the Higuchi constant. 10 The regression analysis was performed and the best fit was found to Higuchi model (table 3) suggesting a release mechanism controlled by diffusion.
The dissolution profiles for different tablets were compared with each other using dissolution efficiency (DE) parameter 11 and the results are showed in table 3.
DISCUSSION
The seasonal NaA extracted from S. cymosum showed different viscosity and the results indicate that polymer viscosity of seasonal NaA samples influenced the release of drug from the matrix tablets. Low viscosity formulation (autumn) exhibited a greater erosion > 90 % of drug release at 1 h, while the drug deliver from the intermediate viscosity samples (winter) showed intermediate drug release (> 90 % after 3 h) and the more viscosity sample (spring) showed more drug retention releasing > 90 % of drug after 6 h. As reported by Efentakis and Koutlis,5 low viscosity polymer results in a more rapid erosion of the tablet and faster release of the drug, compared to high viscosity NaA samples. However, another intermediate viscosity sample (summer) exhibited similar drug release profile to higher viscosity NaA sample, pointing the need of characterization of the alginate samples for the pharmaceutical applications. The behaviour of the intermediate viscosity sample (summer) in the drug release may be affected, beside viscosity, by other factors, as the aggregation state of the polymer, and consequent cohesiveness. The more accuracy physical characterization may be conducted to clarify this point. The Dissolution Efficiency (DE) of batches shows statistical differences, in the order: autumn and winter > summer and spring (table 3). The tablets with more viscous polymers showed lower DE.
All formulations showed the same drug release mechanism, by diffusion (table 3). In the Efentakis and Koutlis,5 study using gelatine capsules and minitablets filled in a hard gelatine capsules containing 69 % of sodium alginate of low, medium and high viscosity, the authors observed a rather zero-order release mechanism, attributed mainly to swelling and an erosion/dissolution process. In the present work the tablets containing 35 % of NaA seasonal samples showed a different mechanism, Higuchi model, probably due to the higher polymer concentration in the tablets. In both studies the viscosity didn't show influence on the drug release mechanism.
ACKNOWLEDGMENTS
The authors wish to thank Medicines Production and Analysis Laboratory - Laboratório de Produção e Análise de Medicamentos (UNIVALI-LAPAM) for providing their facilities, to FAPESC (Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina) and Probic-UNIVALI, for the financial support during this study.
REFERENCES
1 Mafra JrLL, Cunha SR. Avaliação da viabilidade da exploração sustentável de um banco de Sargassum sp. (Phaeophyta) na ilha Feia, Piçarras, SC. XIII Semana Nacional de Oceanografia, Itajai-SC. 2000. p. 542-4.
2. Painter TJ. Algae polysaccharides. In: Aspinall GO. The polysaccharides. New York: Academic Press; 1983. p. 95-285.
3. Haug A, Smidsrod O. The effect of divalent metals on the properties of alginate solutions. I. Calcium ions. Acta Chem Scand. 1965;19:341-51.
4. Bhardwaj TR, Kanwar M, Lal R, Gupta A. Natural gums and modified Natural gums as sustained-release carriers. Drug Dev Ind Pharm. 2000; 26(10):1025-38.
5. Efentakis M, Koutlis A. Release of Furosemide from multiple-unit and single-unit preparations containing different viscosity grades of sodium alginate. Pharm Dev Technol. 2001;6(1):91-8.
6. Hernandéz-Carmona G, Mchugh DJ, Arvizu-Higuera DL, Rodríguez-Montesinos E. Pilot plant scale extraction of alginates from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. J Appl Phycology. 1999;10:507-13.
7. Hernandéz-Carmona G, Mchugh DJ López-Gutiérrez F. Pilot plant scale extraction of alginates from Macrocystis pyrifera. 2. Studies on extraction conditions and methods for separating the alkaline-insoluble residue. J Appl Phycology. 1999;11:493-502.
8. The United States Pharmacopeial Convention. The National Formulary, USP XXVI, NF 21. Rockville: Mack Printing; 2003.
9. Brittain HG. Particle size distribution. Part III Determination by analytical sieving. Pharmaceut Technol North Am. 2002;1:56-64.
10. Higuchi T. Mechanism of sustained-release medication- theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(2):1145-9.
11. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução de Diretoria Colegiada RE nº 310, de 01 de setembro de 2004. Guia para realização do estudo e elaboração do relatório de equivalência farmacêutica e perfil de dissolução. Diário oficial da União, 03 set 2004.
Recibido: 12 de enero de 2009.
Aprobado: 17 de febrero de 2009.
Dr. Itamar Francisco Andreazza. Rua Uruguai, 458, bloco 27, sala 301, centro, CEP 88302-202 CP 360 Itajaí (SC), Brazil. E-mail: itamar@ufpr.br