Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión On-line ISSN 1561-2988
Rev Cubana Farm vol.48 no.4 Ciudad de la Habana oct.-dic. 2014
PRODUCTO NATURAL
Inhibitory effect of Allophylus cominia (L.) Sw leaves aqueous extract on tyrosine phosphatase 1B and dipeptidyl peptidase IV proteins
Efecto inhibitorio de un extracto acuoso de las hojas de Allophylus cominia (L.) Sw sobre las proteínas tirosina fosfatasa 1B y dipeptidil peptidasa IV
DraC. Janet Sánchez Calero,I Lic. Louise C. Young,II DrC. Evangelina Marrero Faz,I Ph.D Alan L HarveyII
I National Centre for Animal and Plant Health (CENSA), San José de las Lajas, Mayabeque, Cuba.
II Strathclyde Institute for Pharmaceutical and Biomedical Sciences (SIPBS) / University of Strathclyde, Glasgow, Scotland, United Kingdom.
ABSTRACT
Introduction: Allophylus cominia (L.) Sw is a Cuban medicinal plant used by traditional medicine for the treatment of diabetes with unknown mechanisms of action.
Objective: to evaluate the effect of Allophylus cominia (L.) Sw leaves aqueous extract and its fractions on protein tyrosine phosphatase 1B (PTP1B) and dipeptidyl peptidase IV (DPPIV) enzymatic activity, as therapeutic targets of type 2 diabetes.
Methods: the aqueous extract of A. cominia leaves was successively partitioned with organic solvents mixtures, thus increasing polarity in order to obtain ten fractions. The extract and its fractions were tested for their possible antidiabetic activity on therapeutic targets of type 2 diabetes: PTP1B and DPPIV. The enzymatic inhibition assays were performed and the inhibitory activity was calculated with the fluorescence values using an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Results: the aqueous extract from A. cominia inhibited the enzymatic activity of PTP1B and DPPIV according to the concentration, being IC50 values equal to 0.69 μg/mL and 344.3 μg/mL, respectively. Several fractions were detected as potent PTP1B inhibitors. The most polar fractions AcF9 and AcF10 were more active, showing IC50 values of 4.4 µg/mL and 3.8 µg/mL respectively. The fractions showed a slight DPPIV inhibition, being fractions AcF6, AcF9 and AcF10 the most active, exhibiting inhibition percentages of 52.0 %, 39.0 % and 40.0 % respectively.
Conclusions: A. cominia aqueous extract and its polar fractions (AcF9 and AcF10) have antidiabetic properties in vitro and are promissory candidates for development of new drugs with inhibitory activity of PTP1B and DPPIV for type 2 diabetes treatment.
Keywords: Allophylus cominia (L.) Sw, antidiabetic activity, PTP1B inhibitors, DPPIV inhibitors, type 2 diabetes therapeutic targets.
RESUMEN
Introducción: Allophylus cominia (L.) Sw es una planta medicinal cubana usada por la medicina tradicional para el tratamiento de la diabetes, cuyo mecanismo de acción es desconocido.
Objetivo: evaluar el efecto del extracto acuoso de hojas de A. cominia (L.) Sw y sus fracciones sobre la proteína tirosina fosfatasa 1B (PTP1B) y dipeptidil peptidasa IV (DPPIV) como diana terapéuticas para el tratamiento de la diabetes tipo 2.
Métodos: el extracto acuoso de hojas de A. cominia fue fraccionado sucesivamente con mezclas de solventes orgánicos, incrementando la polaridad, para obtener diez fracciones. El extracto y sus fracciones fueron evaluados para su posible actividad antidiabética sobre diana terapéuticas de diabetes tipo 2: PTP1B y DPPIV. Se realizaron ensayos de inhibición enzimática y la actividad inhibitoria se calculó a partir de los valores de fluorescencia, empleando longitudes de onda de excitación y de emisión de 360 nm y 460 nm respectivamente.
Resultados: el extracto acuoso de A. cominia inhibió la actividad enzimática de PTP1B y DPPIV de manera dependiente de la concentración, con valores de CI50 de 0,69 μg/mL y 344,3 μg/mL respectivamente. Varias fracciones se detectaron como potentes inhibidores de PTP1B. Las fracciones más polares AcF9 y AcF10 fueron las más activas, y mostraron valores de CI50 de 4,4 µg/mL y 3,8 µg/mL respectivamente. Las fracciones mostraron una ligera inhibición de DPPIV, y las más activas resultaron AcF6, AcF9 y AcF10, con valores de porcentajes de inhibición de 52,0 %, 39,0 % y 40,0 % respectivamente.
Conclusiones: el extracto acuoso de A. cominia y sus fracciones polares (AcF9 y AcF10) tienen propiedades antidiabéticas in vitro y son candidatos promisorios para el desarrollo de nuevos medicamentos con actividad inhibidora de PTP1B y DPPIV para el tratamiento de la diabetes tipo 2.
Palabras clave: Allophylus cominia (L.) Sw, actividad antidiabética, inhibidores de PTP1B, inhibidores de DPPIV, diana terapéuticas de diabetes tipo 2.
INTRODUCTION
Diabetes mellitus is a metabolic disorder of multiple etiologies characterized by chronic hyperglycemia and altered metabolism of lipids, carbohydrates and proteins, due to defects in insulin secretion and /or insulin action.1
Protein tyrosine phosphatase 1B (PTP1B) has been implicated in the negative regulation of insulin signaling by dephosphorylating the insulin receptor (IR) as well as its substrate, insulin receptor substrate-1 (IRS-1) and insulin receptor substrate-2 (IRS-2), and selective inhibition of PTP1B has emerged as a potential drug target for the treatment of type 2 diabetes.2
Dipeptidyl peptidase IV (DPPIV) is a serine protease, which causes breakdown of the peptides that containing proline or alanine as the second residue. The most important substrate for this enzyme are incretin hormones such as: glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are released into the intestine in response to nutrient ingestion and stimulate insulin secretion induced by glucose.3 Thus, GLP-1 stimulates insulin biosynthesis and secretion, reduces glucagon release, slows gastric emptying, reduces appetite and stimulates the regeneration and differentiation of Langerhans islets of β cells.4 Moreover, GIP is involved in glucose metabolism by increasing insulin secretion.5 Both peptides have short half-lives due to rapid degradation by protease DPPIV. DPP-IV inhibitors are a new class of oral anti-hyperglycemic agents, whose effect is mediated through the incretin hormones. Thus, the inhibition of DPPIV enzymatic activity, prolongs the action of GLP-1 and GIP, which maintain glucose homeostasis. Hence, DPP-IV inhibition has the potential to be a novel, efficient and tolerable approach to treat type 2 diabetes.6
Allophylus cominia (L.) Sw (Sapindaceae), also known as Rhus cominia (L.) or Schmidelia cominia Sw, which common name is palo de caja, caja or caja común, is one of the most well-known medicinal plant in Cuba.7 It was initially used as a remedy against gastrointestinal disorders, but was subsequently employed as a remedy for diabetes. It has also been reported in the use of tuberculosis and catarrhal diseases in general. In addition, medicinal properties against toothache and use as a blood purifier in venereal diseases have also been attributed to this plant.7
Several investigations have reported the hypoglycaemic activity of A. cominia aqueous extracts in normoglycaemic and type 1 diabetic animals models.8-10
The phytochemical studies of the aqueous extract of leaves from A. cominia revealed the presence of tannins, free amines, phenols, triterpenes and steroids. In addition, in this extract proteins, carbohydrates such as arabinose, xylose, galactose, glucose and fatty acids (lauric, miristic, palmitic, estearic and arachidonic) were identified.11
The aim of the study was to investigate the inhibitory effect on PTP1B and DPPIV of A cominia leaves (aqueous extract and its fractions) as the underlying mechanism to the potential type 2 anti-diabetic properties of this plant.
METHODS
PLANT MATERIAL
Allophylus cominia (L.) Sw (Sapindaceae) leaves were collected from forest of Cotilla (San José de Las Lajas, Mayabeque, Cuba) in the month of February (2008). Plants were taxonomically authenticated by Prof. Fernando Franco Flores, in the Laboratory of Botany at the Agriculture University of Havana, Cuba. A voucher specimen of the plant is kept for reference (HFA-1769) in the Herbarium of this institution.
PLANT EXTRACT
Fresh leaves of A. cominia were dried in a stove at 37 °C for 96 h. The dry leaves were milled in fine particles of 5 mm. The plant extract was obtained from powder aqueous extraction at 95 °C for 30 min, in a 10 % (w/v) relation. The resulting extract was freeze dried. Dry extract was stored in plastic bottles in a desiccator for future experiments.
FRACTIONATION OF PLANT EXTRACT
Ten fractions were obtained from A. cominia aqueous extract using a Flash Chromatography System. It was used 5 g of dried extract in adsorption chromatography employing a column (Isolute Flash, Si, 100 g) and as mobile phase, an organic solvents mixture, from low to high polarity (n-hexane; 1,2 di-chloro-metane; 2-butanol; methanol and water). Elution was achieved using solvent gradients of 400 mL aliquots of n-hexane 100 % (AcF1), 1,2 di-chloro-metane 100 % (AcF2), 1,2 di-chloro-metane 75 %: 2-butanol 25 % (AcF3), 1,2 di-chloro-metane 50 %: 2-butanol 50 % (AcF4), 1,2 di-chloro-metane 25 %: 2-butanol 75 % (AcF5), 2-butanol 100 % (AcF6), 2-butanol 75 %: methanol 25 % (AcF7), 2-butanol 50 %: methanol 50% (AcF8), 2-butanol 25 %: methanol 75 % (AcF9) and methanol 50 %: water 50 % (AcF10).
PROTEIN TYROSINE PHOSPHATASE 1B (PTP1B) ENZYME ASSAY
The assay was performed as previously described, in 96-well black microplates.12 PTP1B (human, recombinant, Sigma) was incubated in the presence and absence of A. cominia extract and its fractions.
For inhibition assay, 25 L of different concentrations of A. cominia aqueous extract and its fractions (0.01-300 µg/mL), the standard inhibitor [Bis(4-Trifluoromethylsulfonamidophenyl)-1,4-diisopropylbenzine] (TFMS, Calbiochem) (0.1-300 µM) or buffer solution (pH 7.2), containing the following reagents at final concentrations indicated: Hepes (25 mM), sodium chloride (50 mM), Dithiothreitol (2 mM), ethylene-diamine-tetraacetic acid (EDTA) (2.5 mM) and Bovine Serum Albumin (BSA) (0.01 mg/mL), were pre-incubated with 50 µL of enzyme for 30 min at 37 ºC. Subsequently, 25 µL of substrate (6.8-difluoro-4-methylumbelliferyl phosphate) (DiFMUP, Invitrogen Ltd.), at a final concentration of 10 µM, was added to the reaction mixture and was placed in a 37 ºC incubator for 10 min. The amount of end product (umbelliferone) obtained was measured by determining change in fluorescence on a Wallac Victor 2 (Perkin-Elmer, Sunnyvale, CA) using an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
DIPEPTIDYL PEPTIDASE IV (DPPIV) ENZYME ASSAY
The assay was performed as previously described, in black 96-well microtitre plates.13 DPPIV (human, recombinant, Sigma) was incubated in the presence and absence of A. cominia extract and its fraction.
For inhibition assays, 25 µL of different concentrations of aqueous extract from A. cominia (0.01-300 µg/mL) and its fractions (100 µg/mL), the standard inhibitor [(3N-[(2S, 3S)-2-amino-3-methyl-pentanoyl]-1,3-thiazolidine) hemifumarate] (P32/98) (0.1-300 µM) or buffer solution (pH 8.00), containing the following chemicals at final concentrations indicated: Tris-HCl (100 mM) with Bovine Serum Albumin (BSA) (0.1 mg/mL), were preincubated with 50 µL of enzyme (final concentration: 0.3 nM) for 30 min at 37 ºC. After 25 mL of substrate [Gly-Pro-7-amido-4-methylcoumarin hydrobromide] (Gly-pro-AMC), at final concentration of 30 µM, was added to reaction mixture and it was placed in a 37 ºC incubator for 30 min. The amount of end product (umbelliferone) obtained was estimated by measuring change in fluorescence on a Wallac Victor 2 (Perkin-Elmer, Sunnyvale, CA) using an excitation wavelength of 355 nm and an emission wavelength of 460 nm.
The percentage inhibition of PTP1B and DPPIV was calculated as follows: [Enzyme in the presence of compound (random fluorescent units)/Enzyme in the absence of compound (random fluorescent units)]*100.
STATISTICAL ANALYSIS
The IC50 value for each compound was calculated by non-linear regression analysis using GraphPad Prism version 4.03 -2005 (La Jolla, California, USA) subsequently, the apparent Ki of the substrate was calculated for each sample using the Cheng-Prusoff equation. All the values were expressed as mean ± standard error of mean (SE) for 4 replicates of the experiments.
Conflict of interest
The authors have no conflict of interest to declare.
RESULTS
A. cominia aqueous extract inhibited the enzymatic activity of PTP1B in a concentration dependent manner. The mean inhibitory concentration (IC50) values was 0.69 µg/mL (Ki 0.26 (± 0.025 µg/mL) (Fig. 1).
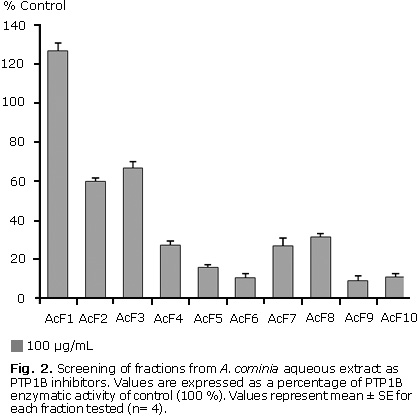
Ten fractions from the A. cominia aqueous extract were screened against PTP1B; seven were identified as possible PTP1B inhibitors. Figure 2 shows that when tested at a concentration of 100 µg/mL (n= 3), fraction 1 appeared to have no effect on the activity of this enzyme, fractions 2 and 3 showed only a small inhibition of PTP1B activity. Fractions 4, 7 and 8 exhibited a moderate inhibition of this protein, whereas fractions 5, 6, 9 and 10 showing the most apparent potent effect on PTP1B inhibition. The percentages of inhibition values in decreasing order: F9 (90.7 %), F6 (89.3 %), F10 (88.7 %), F5 (84.0 %), F7 (72.9 %), F4 (72.7 %), F8 (68.3 %), F2 (40.0 %), F3 (33.0 %).
Figure 3 (A, B, C, D, E and F) shows dose-response curves of the more active fractions, obtained from the aqueous extract of A. cominia leaves, on enzymatic activity of PTP1B. The graphs show that PTP1B inhibitory activity increased in a dependent manner of the fractions concentration, reaching inhibition values close to 100 % at the maximum concentration tested (300 µg/mL). Differences were found in the IC50 values among different fractions. The more active fractions, AcF9 and AcF10, appeared to be related to increasing polarity, which gave IC50 values of 4.4 µg/mL and 3.8 µg/mL (Ki 1.7 and 1.4 µg/mL) respectively.
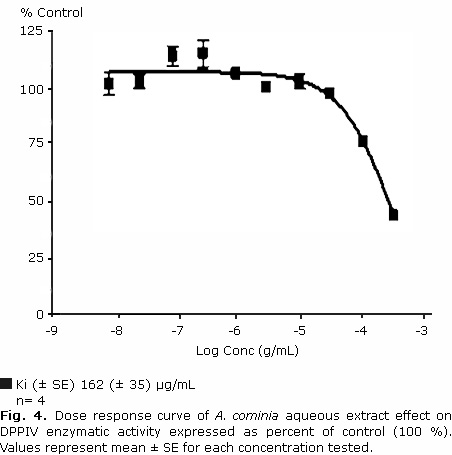
A. cominia aqueous extract inhibited the enzymatic activity of DPPIV in a concentration dependent manner, reaching 50 % percentage of inhibition values around the highest concentration tested of 300 μg/mL (Fig. 4).
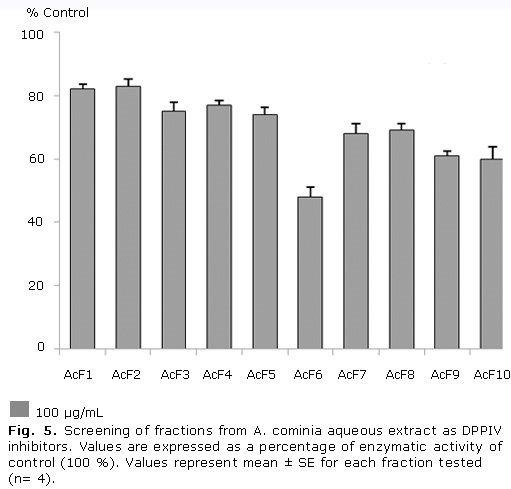
Figure 5 shows that fractions 6, 9 and 10 exhibited moderate inhibition of DPPIV activity, resulting fraction 6 the most active fraction in this assay; while the other fractions showed a slight inhibition of this protein. The percentage of inhibition values in decreasing order were: F6 (52.0 %), F10 (40.0 %), F9 (39.0 %), F7 (32.0 %), F8 (31.0 %), F5 (26.0 %), F3 (25.0 %), F4 (23.0 %), F1 (18.0 %), F2 (17.0 %).
DISCUSSION
The PTP1B and DPPIV inhibition assays were used in the present study to elucidate the type 2 antidiabetic mechanism of action of the A. cominia aqueous extract.
In line with the present research, other plant extracts have shown inhibitory effect on PTP1B enzymatic activity in the same range of concentrations as the A. cominia aqueous extract. Thus, the methanolic extract of Psidium guajava displayed a significant inhibitory effect of 87 % against PTP1B at a concentration of 30 μg/mL.14 Other authors have reported that the methanolic extract of dried roots of Salvia miltiorrhiza exhibited a significant inhibitory effect on the activity of this enzyme.15
The differences in the polarity of the solvents used in the mobile phase during fractionation process of A. cominia extract tends to bring out varieties of compounds in the diverse fractions. Hence, the inhibitory activity on PTP1B across the fractions indicates that different types of compounds are able to act as inhibitors of this enzyme.
It has also been previously reported that PTP1B can be inhibited by different types of secondary metabolites from plants. A study reported that several types of compounds isolated from the plant Ardisia japonica: oleanolic acid, quercitrin and norbergenin showed a moderate inhibitory activity on this enzyme.16 Other authors have reported that three diterpene compounds obtained from methanolic extract of dried roots of Salvia miltiorrhiza as responsible for this effect which exhibited noncompetitive inhibition of PTP1B activity with IC50 values of 11.4 ± 0.6 mM, 22.4 ± 0.6 mM and 56.1 ± 6.3 mM, respectively.15 Additional work found that triterpenes isolated from the methanol extract of Diospyros kaki (Persimmon) leaves had an inhibitory effect on the activity of PTP1B enzyme with IC50 values in the range of 3.1 ± 0.2 to 18.8 ± 1.3 mM.17 It was also reported that a proteoglycan obtained from the fruit of Ganoderma lucidum, showed efficient PTP1B inhibitory potency with an IC50 value of 5.12 ± 0.05 μg/mL.2
The fraction 6 (butanolic fraction) from A. cominia resulted one of the more active fraction in the PTP1B inhibition. This result correspond with data refered in other papers, which informed that the butanolic fraction from methanolic extract of Psidium guajava containing the active entity with an IC50 value of 2.6 μg/mL.14 Unpublished studies demonstrated recently the AcF6 contains flavonoids, which are the secondary metabolites responsible of the PTP1B inhibition. Thus, quercitrin and norbergenin isolated from Ardisia japonica showed a moderate inhibitory activity on this enzyme with IC50 values of 24 μM and 28 μM respectively.16
The fractions 9 and 10 were the most active fractions in this assay. Unpublished results about chemical characterization of them indicated that contained tannins, which was previously demonstrated for A. cominia aqueous extract,11 although further experiments are necessary in order to dilucidated their chemical structure. Other authors confirm the importance of tannins in the PTP1B inhibitory effect, comparing results of total methanolic extract and detannificated extract of Cichorium intybus, demonstrated that tannins are responsible component of this pharmacological effect.18
Previous studies have shown that PTP1B enzyme has two active sites: a catalytic site and an allosteric site. X-ray diffraction has identified the interactions between PTP1B and allosteric inhibitors.19 A general mechanism proposed for other proteins tyrosine phosphatases is that allosteric inhibitors prevent the formation of the enzymes active form, blocking the WPD loop mobility in the catalytic site, which closure is essential for catalytic activity of the enzyme.20 Hansen, et al., 2005 reported that the allosteric inhibition of PTP1B activity can be achieved by selective modification of cysteine residues, Cys 121, which although not located in the catalytic site, providing interactions with residues which are in contact with the His 214, which has been shown to be important for catalysis.21 Thus, the amentoflavone obtained from Selaginella tamariscina, a biflavonoide, inhibited PTP1B activity by allosteric inhibition.19 Further work to determine whether the potentially active fractions of A. cominia are working at the catalytic or an allosteric site.
Consistent with the results in this study, many other papers have reported inhibition of DPPIV enzymatic activity by plant extracts: the aqueous extract from Cistus incanus L. leaves,22 the methanolic extract of Mangifera indica leaves,23 the aqueous extracts of plants native of Armenia which cited cloves, cinnamon, green and black tea being highly effective, blackberry leaves, melilot, oregano and sea-buckthorn which behaved as inhibitors of this enzyme, in combination with other antidiabetic drugs.24 In addition, the hexane extract of Annona squamosa inhibited DPP-IV at 30 mg/mL.25
DPP-IV is a 766-amino acid transmembrane glycoprotein consisting of three parts, a cytoplasmic tail, a trans-membrane region and an extracellular part. The extracellular part is divided into a catalytic domain and an eight-bladed β-propeller domain. The latter contributes to the inhibitor binding site which consists of a deep lipophilic pocket combined with several exposed aromatic side chains achieving high affinity for small molecule binding. DPP-IV inhibitors usually have an electrophilic group that can interact with the hydroxyl of the catalytic serine in the active binding site. DPP-IV inhibitors without the electrophilic group have shown toxicity due to affinity to other dipeptidyl peptidases, such as: DPP-2, DPP-8 and DPP-9.26
The secondary metabolites responsible of the DPPIV inhibition may be the flavonoids present in the A. cominia aqueous extract which existence was recently demonstrated in unpublished studies. This type of compound probably is present in the most active fraction (AcF6), but further experiments are necessary for confirmation it. Thus, an article describes the antidiabetic effect of the flavonoid rich fraction of Pilea microphylla (L.) inhibited dipeptidyl peptidase IV (DPP-IV) in vitro with an IC50= 520.4 ± 15.4 μg/mL.27
Further studies will be necessary in order to corroborate these effects on in vivo models of type 2 diabetes. Also it will be important to approach toxicological studies of the active fractions in order to demonstrate the safety of them.
CONCLUSION
In our in vitro research, A. cominia aqueous extract, in particular its polar fractions (AcF9 and AcF10) have promissory antidiabetic properties mediated by PTP1B and DPPIV, relevant therapeutic targets for type 2 diabetes.
Acknowledgements
This work was supported by an Academic Link from British Council and an IFS research project No. F/4906-1.
REFERENCES
1. American Diabetes Association (ADA). Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012;35:64-71.
2. Teng BS, Wang CD, Yang HJ, Wu JS, Zhang D, Zheng M, et al. A Protein Tyrosine Phosphatase 1B activity inhibitor from the fruiting bodies of Ganoderma lucidum (Fr.) Karst and its hypoglycemic potency on streptozotocin-induced type 2 diabetic mice. J Agric Food Chem. 2011;59:6492-500.
3. Mentlein R, Gallwitz B, Schmidt W. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829-35.
4. Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653-9.
5. Meier JJ, Nauck MA, Schmidt WE, Gallwitz B. Gastric inhibitory polypeptide: The neglected incretin revisited. Regul Pept. 2002;107:1-13.
6. Havale SH, Pal M. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg Med Chem. 2009;17:1783-802.
7. Roig JT. Plantas medicinales, aromáticas y venenosas de Cuba. 2da ed. La Habana: Editorial Científica Técnica; 1988.
8. Melchor G, García L, Marrero E, Lorenzo L. Actividad hipoglicemiante oral de Allophylus cominia L. Sw (palo de caja) en ratas normoglicémicas. Rev Salud Anim. 1999;21:35.
9. Valls J, Véliz T, Marrero E, Lagunas A. Evaluación farmacológica de diferentes extractos obtenidos a partir de la especie vegetal Allophylus cominia (L.) Sw. Rev Cubana Farm. 2000;34:82-3.
10. Véliz T. Efecto del extracto acuoso de Allophylus cominia (L.) Sw en modelos roedores in vivo y ex vivo. Tesis de Master en Farmacología. IFAL. Universidad de La Habana, La Habana, Cuba, 2001.
11. Véliz T, Valls J, Sanchez LM, Noa M, Marrero E. Detection and determination of chemical groups in an extract of Allophylus cominia (L.). J Herb Pharmacother. 2005;5:31.
12. Montalibet J, Skorey KI, Kennedy BP. Protein tyrosine phosphatase: enzymatic assays. Methods. 2005;35:2-8.
13. Cooper KG, Woods JP. Secreted Dipeptidyl Peptidase IV activity in the dimorphic fungal pathogen Histoplasma capsulatum. Infect Immun. 2009;77:2447-54.
14. Oh WK, Lee CH, Lee MS, Bae EY, Sohn CB, Oh H, et al. Antidiabetic effects of extracts from Psidium guajava. J Ethnopharmacol. 2005;96:411-5.
15. Yu H, Hyuncheol O, Minkyun N, Beom K, Won O, Bo K, et al. PTP1B inhibitory effect of Abietane Diterpenes isolated from Salvia miltiorrhiza. Biol Pharm Bull. 2005;28:1795-7.
16. Yan-Fang L, Li-Hong H, Feng-Chang L, Jia L, Qiang S. PTP1B inhibitors from Ardisia japonica. J Asian Nat Prod Res. 2005;7:13-8.
17. Thuong PT, Lee CH, Dao TT, Nguyen PH, Kim WG, Lee SJ, et al. Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J Nat Prod. 2008;71:1775-8.
18. Muthusamy VS, Anand S, Sangeetha KN, Sujatha S, Arun B, Lakshmi BS. Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem Biol Interact. 2008;174:69-78.
19. Lee JY, Jung KW, Woo ER, Kim Y. Docking Study of Biflavonoids, Allosteric Inhibitors of Protein Tyrosine Phosphatase 1B. Bull Korean Chem Soc. 2008;29:1479.
20. Wiesmann Ch, Barr KJ, Kung J, Zhu J, Erlanson DA, Shen W, et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol. 2004;11:730-7.
21. Hansen SK, Cancilla MT, Shiau TP, Kung J , Chen T, Erlanson DA. Allosteric Inhibition of PTP1B Activity by Selective Modification of a Non-Active Site Cysteine Residue. Biochemistry. 2005;44:7704-12.
22. Lendeckel U, Arndt M, Wolke C, Reinhold D, Kahne T, Ansorge S. Inhibition of human leukocyte function, alanyl aminopeptidase (APN, CD13) and dipeptidylpeptidase IV (DP IV, CD26) enzymatic activities by aqueous extracts of Cistus incanus L. ssp. Incanus. J Ethnopharmacol. 2002;79:221-7.
23. Yogisha S, Raveesha KA. Dipeptidyl Peptidase IV inhibitory activity of Mangifera indica. J Nat Prod. 2010;3:76-9.
24. Mardanyan S, Sharoyan S, Antonyan A, Zakaryan N. Dipeptidyl peptidase IV and adenosine deaminase inhibition by Armenian plants and antidiabetic drugs. Int J Diabetes Metab. 2011 [citado 13 feb 2013];19(2). Disponible en: http://ijod.uaeu.ac.ae/iss_1902/d.htm
25. Davis JA, Sharma S, Mittra S, Sujatha S, Kanaujia A, Shukla G, et al. Antihyperglycemic effect of Annona squamosa hexane extract in type 2 diabetes animal model: PTP1B inhibition, a possible mechanism of action? Indian J Pharmacol. 2012;44:326-32.
26. Green B, Flatt P, Bailey C. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc Dis Res. 2006;3:159-65.
27. Pannakal ST. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoid rich fraction of Pilea microphylla (L.) in high fat diet/streptozotocin-induced diabetes in mice. Exp Toxicol Pathol. 2011;63:203-6.
Recibido: 21 de julio de 2014.
Aprobado: 28 de agosto de 2014.
Janet Sánchez Calero. Centro Nacional de Sanidad Agropecuaria (CENSA). Carretera de Tapaste y Autopista Nacional. San José de las Lajas. Mayabeque. Cuba. CP 32700.E-mail: jsanchez@censa.edu.cu.