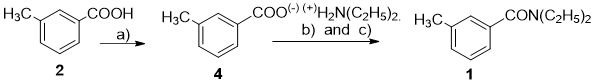INTRODUCTION
N,N-diethyl-m-methylbenzamide or N,N-diethyl-m-toluamide (DEET)(1) was synthesized in 1944 and used in army as insect repellent.1) Until now, DEET became the most popular reagent for insect repellent because of its effects in preventing insect attacks which causes many fatal diseases such as Malaria, Zika, viral hemorrhagic fever, etc.
Thus far, DEET could be synthesized by many methods. Among them, the most common is the acylation of diethylamine with m-toluic acid deriving its halogenation with thionyl chloride, oxalyl chloride or phosphorus chloride.
Numerous papers and patents regarding synthetic method of N,N-diethyl m-methylbenzamide (1) started from various input materials such as m-toluic acid, m-toluoyl chloride, butyl m-toluoate, m-toluyl-nitrile, m-xylene, etc. with diethylamine.
The essential nature of reaction for preparation of N,N-diethyl-m-methylbenzamide (1) from m-toluic acid derivatives and diethylamine is m-toluoylation, the link H-N of diethylamine. However, the m-toluic acid (2) is a weak m-toluoylation agent, therefore cannot easily derivate m-toluoylated diethylamine into N,N-diethyl-m-methylbenzamide. This process creates diethylammonium m-toluolate, the salt of diethylamine and m-toluic acid. DEET could be synthesized by dehydration of that salt under strict conditions,2,3,4,5 such as in the presence of expensive catalysts and high temperature and/or pressure, that causes dark color of DEET product, which require other step to purify it using vacuum distillation or column chromatography (Fig. 1).
To replace the above process, m-toluic acid often is activated into m-toluoyl chloride (3) by chlorinating agents. The most common method for synthesis of DEET is acylation of diethylamine with m-toluic acid (2) or m-toluoyl chloride (3),6,7 prepared from m-toluic acid and a chlorinating agents,8,9,10,11,12,13,14 such as thionyl chloride,8,9,10,11 oxalyl chloride,10 phosphorus trichloride,12 niobium pentachloride,13 2,2,2-trichloro-acetamide,14 that are highly toxic reagents (Fig. 2).
Nonetheless, above listed methods for preparation of DEET have a number of common deficiencies, such as the synthesis of 1 from m-toluic acid and diethylamine via intermediate m-toluoyl chloride (3), which was prepared from m-toluic acid by treatment with chlorinating agents that are highly toxic reagents, while dehydration of compound 4 into 1 must be carried out at conditions of high temperature; and finally the purification of 1 must use vacuum distillation or column chromatography. Besides, the total preparation time of that processes is long. and also lead to lower economic yield. Therefore, the above procedures are feasible for industrial scale.
Reagents and conditions:
a) Chlorinating reagents, solvent;
b) HN(C2H5)2, solvent;
c) Vacuum distillation or column chromatography.
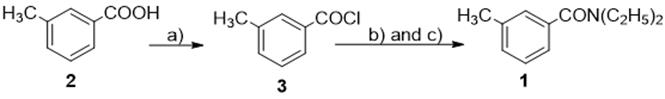
Fig. 2 Preparation of DEET by acylation of diethylamine with the m-toluyl chloride.8,9,10,11,12,13,14
Reagents and conditions:
a) HN(C2H5)2, solvent;
b) Solvent, high temperature, catalyst;
c) Vacuum distillation or column chromatography.
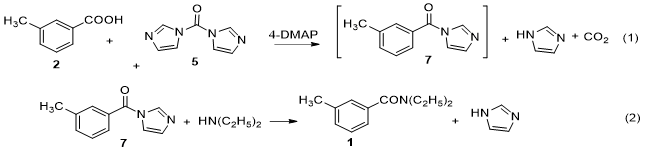
Accordingly, we have chosen this method as a new and feasible method for synthesis of DEET (Fig. 3). Besides, the parameters of this procedure were optimized to produce the best condition. Our goal was to increase the effectiveness of the procedure, carrying it out at low temperature in one-pot, with relative higher overall yield and limited use of toxic reagents and solvents, as well as no necessary purification by vacuum distillation or column chromatography.
Reagents and conditions:
METHODS
We have tried to screen all factors affecting the yield of N,N-diethyl-m-toluamide, to establish the suitable reaction conditions which can apply to laboratory scale.
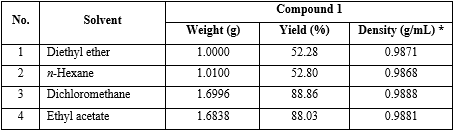
1. Effect of solvent types on the yield of (1):
Solution of m-toluic acid (2) (1.36 g, 0.01 mol), 1,1’-carbonyl-diimidazole (1.62 g, 0.01 mol) and 4-dimethylamino-pyridine (0.037 g, 0.0003 mol) in dried dichloromethane (5 mL) was stirred at room temperature, then heated to 35-40 oC and to reflux until the compound 2 completely disappeared (for 0.5 hour; indicated by thin layer chromatography (TLC)); solvents: n-hexane: C2H5OAc, ratio 1:1 volume/volume (v/v); visualization: UV lamp of 254 nm). Then diethylamine (3.1 mL, 0.03 mol) was added and the mixture heated to 35-40 oC until the compound 7 completely disappeared (1 h, the reaction was monitored by TLC, n-hexane: EtOAc = 1:1, v/v; detection by UV of 254 nm).
After the reaction completion, the mixture was cooled to 15-20 oC and to this mixture, dichloromethane (5 mL) was added and adjusted to pH 9-10 with 5 % sodium hydroxide (3 mL) with stirring. The dichloromethane layer was separated and washed with cooled water; the organic layer was adjusted to pH 5-6 with aq. 10 % HCl (7 mL). The dichloromethane was separated and washed with distilled cold water (8 mL x 2). The organic layer was dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum at 35-40 oC and dried under vacuum at 20oC/1 mmHg for 2 h to give 1.6996 g DEET (1) (88.86 %), as a slight yellow liquid; purity (HPLC): 95-97%.
The reaction preparation of (1) was performed with the same above operation, but instead solvent type were diethyl ether, n-hexane, dichloromethane and ethyl acetate (table 1).
Reaction parameters: m-toluic acid (2) = 0.01 mol; solvent type, instead of diethyl ether, n-hexane, dichloromethane, ethyl acetate; molar ratio (compound 1: CDI: diethylamine) = (1:1:3). Density (g/mL)* was calculated by weighting 1 mL final product at 20 oC.
Conclusion: The solvent that gives the best yield of 1 was dichloromethane (yield 88.86 %) (No. 3 in table 1).
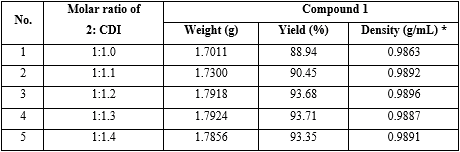
2. Effect of molar ratio between compound 2 and CDI on the yield of compound (1):
Experiment: The reaction preparation of 1 was performed with the same operation as investigation on the effect of solvent type on the yield of 1, but solvent was dichloromethane; molar ratio between compound 2 and CDI was 1:1.0; 1:1.1; 1:1.2; 1:1.3 and 1:1.4, respectively (table 2).
Reaction parameters: Solvent was dichloromethane; total reaction time = 90 minutes; molar ratio of (compound 2: diethylamine) = (1:3). Molar ratio between compound 2 and CDI was 1:1.0; to 1:1.4, respectively.
Conclusion: The results found that using molar ratio of compound 2 and CDI 1:1.2 got the highest yield of 1, 93.68 % (No. 3 in table 2).
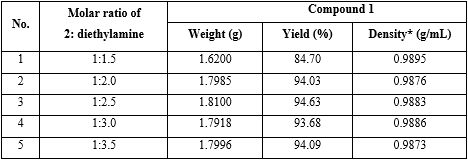
3. Effect of molar ratio between compound 2 and diethylamine on the yield of 1:
Experiment: The reaction preparation of 1 was performed with the same operation as investigation on the effect of molar ratio between compound 2 and CDI on the yield of 1, but molar ratio between compound 2 and CDI was 1:1.3, and molar ratio between compound 2 and diethylamine was 1:1.5; 1:2.0; 1:2.5; 1:3.0 and 1:3.5, respectively (table 3).
Reaction parameters: Solvent was dichloromethane; total reaction time = 90 minutes; molar ratio of (compound 2: CDI) = (1:1.2), and molar ratio of (compound 2: diethylamine) from 1:1.5 to 1:3.5, respectively.
Conclusion: The result found that using molar ratio of compound 2 and diethylamine 1:2.0 got the highest yield, 94.03 % (No. 2 in table 3).
The combination of reaction parameters found that the highest yield of DEET (1) were the following: m-toluic acid (2) = 0.01 mol; reaction condition: total reaction time = 90 minutes; molar ratio of (compound 2: CDI: diethylamine) = (1:1.2:2.0); yield = 94.03 %.
RESULTS
Based on the optimal conditions, we have applied and scaled to synthesize the final compound (DEET).
A solution of m-toluic acid (2) (340.39 g, 2.5 mol), 1,1’-carbonyl-diimidazole (486.46 g, 3.0 mol) and 4-dimethylamino-pyridine (9.16 g, 0.075 mol) in dried dichloromethane (1250 mL) was stirred at room temperature and then heated to 35-40 oC to reflux, until the compound (2) completely disappeared (for 0.5 h, indicated by TLC, solvents: n-hexane: C2H5OAc = 1: 1, v/v; visualization: UV lamp of 254 nm), then diethylamine (520 mL, 5.0 mol) was added and heated to 35-40 oC until the intermediate compound 7 completely disappeared using TLC to follow.
After the completion of reaction, the mixture was cooled to 15-20 oC and mixed with dichloromethane (1250 mL), then adjusted to pH 9-10 with 5 % sodium hydroxide (750 mL) with stirring. The dichloromethane layer was separated and washed with cold water; the organic layer was adjusted to pH 5-6 with aq. 10 % HCl (1750 mL). The dichloromethane was separated and washed with distilled cold water (2000 mL x 2). The organic layer was dried over anhydrous sodium sulfate. The solvent was evaporated under vacuum at 35-40oC and dried under vacuum at 20oC/1 mmHg for 2 h, to give 457.53 g DEET (1) (95.68 %), as a weak yellow liquid.
Analytical data: The purity of final product was checked using high-performance liquid chromatography (HPLC) system: 95-97 %; Infrared spectroscopy (IR) (KBr, νcm-1): 2970-2873 (C-H), 1625 (C= O), 1215 (C-N). Mass spectroscopy (MS): found 191.8 [M], calculated: 191.27. Nuclear magnetic resonance (NMR) data using solvent dimethyl sulfoxide deuteride (DMSO-d6):1) H-NMR (500MHz, DMSO-d6) δ (ppm) 7.29 (triplet, 1H, ArH, J = 10 Hz), 7.22 (doublet, 1H, ArH, J = 5Hz), 7.13 (singlet, 1H, ArH), 7.11 (doublet, 1H, ArH, J = 7 Hz) 3.42 (singlet, broad, 2H, CH2), 3.17 (singlet, broad, 2H, CH2), 2.32 (singlet, 3H, CH3-Ar), 1.12 (singlet, broad, 3H, CH3) 1.04 (singlet, broad, 3H, CH3).13) C-NMR (125MHz, DMSO-d6) δ (ppm) 169.96 (C=O), 137.6 (C3-Ar), 137.3 (C1-Ar), 129.4 (C4-Ar), 128.1 (C5-Ar), 126.5 (C2-Ar), 122.9 (C6-Ar) 42.7 (CH2), 38.56 (CH2), 20.8 (CH3-Ar), 13.9 (CH3-CH2), 12.7 (CH3-CH2).
DISCUSSION
We demonstrate that (Fig. 3) 1 can be synthesized from m-toluic acid (2) and diethylamine, in the presence of 1,1’-carbonyl-diimidazole (5) and 4-dimethylaminopyridine (4-DMAP (6)) in dichloromethane. With two reactions in one-pot via high activity of intermediate compound 1-(m-toluoyl) imidazole (7) which did not require the separation and purification. Especially in this study, all byproducts were water-soluble, easily removed in aqueous workup with the liquid-liquid extraction method (water-dichloromethane). Most of DEET obtained was of exceptional purity, therefore was not necessary to purify it by vacuum distillation or column chromatography, as reported earlier.
The procedure for preparation of DEET from m-toluic acid (2), 1,1’-carbonyl-diimidazole CDI,5 in the presence of 4-DMAP (6) as catalyst via intermediate 1-(m-toluoyl) imidazole (7) and diethylamine has shown in figure 4.
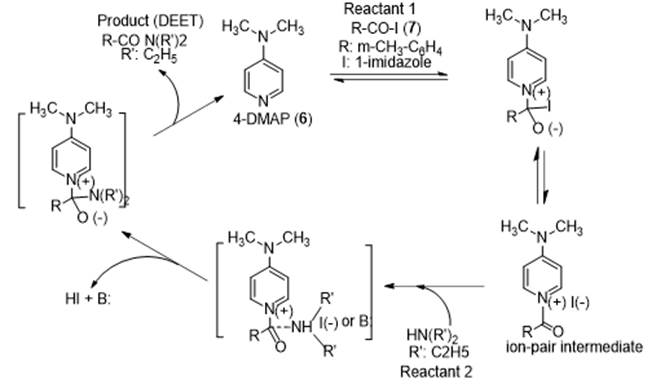
The 4-DMAP catalyzed acylation of diethylamine by 1-(m-toluoyl) imidazole (R-CO-I, I: imidazole is currently believed to proceed via the nucleophilic catalysis mechanism, shown in figure 5. This mechanism is supported by following observations:
The above synthesis procedure has the following advantages:
a) Highly toxic reagents and solvents were not used;
b) The conversion of 2 to 1 via intermediate 1-(m-toluyl) imidazole (7) was carried out at low temperature (35-40 oC);
c) The separation and purification of the intermediate 7 was not necessary, it led to a decrease in the total time of the process and increased the overall performance of the DEET preparation process;
d) The separation and purification of DEET by vacuum distillation or column chromatography was not necessary, and was found that one-pot synthesis of DEET was operationally straight, and with all byproducts easily removed in aqueous workup with the liquid-liquid extraction method (water-dichloromethane);
e) The parameters of reaction as the method for execution of reaction, the reaction temperature, the reaction time, the molar ratio of reagents, the solvents type, solvent volume, the method for execution of reaction, the method for isolation, purification of product 1 was also examined (as shown in table 1, table 2 and table 3);
f) Total preparation time of 1 from 2 was shorter. As a result, we have set up the new, feasible method for synthesis of N,N-diethyl m-methyl-benzamide (1) with more advantages than the previous reported, including simplicity of synthesis procedure; high synthesis yield 94-95 % compared with 51-85 % in previous publications.