Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión On-line ISSN 1819-4087
cultrop vol.40 no.2 La Habana abr.-jun. 2019 Epub 01-Jun-2019
Original Article
Germination and development of Rhizoglomus sp. propagules in vitro
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
The physiological studies related to the mycorrhizal symbiosis have made difficult due to the condition of the symbionts that are characterized by the fungi that intervene in the association, which, up to now, can’t grow in axenic conditions. However, in vitro root cultivation techniques, as a simplified system for the establishment of the arbuscular mycorrhizal symbiosis, have been widely used and they are very important in several investigations. The aim of this study was to obtain disinfected spores of Rhizoglomus sp. (INCAM-11) from soil, which express high percentages of germination and low levels of contamination on in vitro conditions and determine the growth period of the germination hyphae. INCAM-11 propagules were disinfected and their percentages of germination and contamination, as well as the germinative tube length were evaluated. Results show that the immersion time of spores in antibiotic solution during 24 hours, before its inoculation in the Modified Strulla and Romand (MSR) medium, can reduces the contamination percentage and the moment of germination. In the other hand, the hyphae germination spreads in the culture medium during 30 days approximately, before stopping their growth, contributing to the later establishment of the association in the transformed roots.
Key words: arbuscular mycorrhizas; disinfection; hypha
INTRODUCTION
Physiological studies related to arbuscular mycorrhizal symbiosis are considerably difficult due to the condition of obligate symbionts that characterize the fungi involved in the association, which, so far, makes it impossible to grow in axenic conditions 1. However, the use of in vitro root culture as a simplified system to establish such symbiosis has been widely used and particularly useful in numerous physiological studies that include two fundamental aspects: the exchange of signals between symbionts and fungal metabolism 2,3.
Although there are currently different species or strains of arbuscular mycorrhizal fungi (AMF) that can survive in the environment provided by this culture system, about 100 1 are reported in the literature, there are still numerous isolates that resist to colonize the transformed roots and even to germinate in those conditions. The continuous methodological improvement of the system, taking into account the composition of the culture media, the growth conditions and the propagules used, will allow many of these species to be successfully cultivated in different hosts and culture systems 4.
In Cuba, the development of environmentally friendly inoculants is part of the state's policy to achieve clean productions and increase the availability of food for the population. Consequently, the National Institute of Agricultural Sciences (INCA) leads the production of inoculants based on arbuscular mycorrhizal fungi (AMF). For this reason, having a certified strain of AMF species in vitro is a priority of the research carried out in the institution.
Therefore, the present study set out the objectives of obtaining disinfected spores of Rhizoglomus sp. (INCAM-11) from soil expressing high germination percentages and low levels of contamination, as well as determining the growth period of the propagation germination hyphae of Rhizoglomus sp. (INCAM-11) under in vitro conditions.
MATERIALS AND METHODS
Biological material
The INCAM-11 strain of Rhizoglomus sp., From the AMF collection of the National Institute of Agricultural Sciences (INCA) of Cuba, was used. The propagules used were isolated from the inoculant through the technique of wet and decanted sieving and subsequent extraction by centrifugation in sucrose gradient+Tween 80 at 2000 rpm for five minutes 5. Once the water-sucrose+Tween 80 interface was obtained, the spores were extracted using a 30 mL syringe.
The SRM medium was used which was composed of (g L-1): Macroelements (MgSO4.7H2O-73.9; KNO3-7.6; KCl-6.5; KH2PO4-0.41; Ca (NO3) 2.4 H2O-35.9; NaFeEDTA-0.16); Microelements (MnSO4.4H2O-1.225; CuSO4.5H2O-1.1; ZnSO4.7H2O - 0.14; H3BO3 - 0.925; Na2MoO4.2H2O - 0.12; (NH4) 6Mo7O24.4H2O - 1.7); Vitamins (Calcium pantothenate-0.09; Biotin-0.0001; Nicotinic acid-0.1; Pyridoxine-0.09; Thiamine-0.1 and Cyanocobalamin-0.04); Sucrose-10). The pH was adjusted to 5.5 before adding 4 g L-1 GellamGum and the medium was sterilized at 121 °C for 15 min.
Spore disinfection of Rhizoglomus sp. (INCAM - 11)
To disinfect the propagules of INCAM-11, the methodology proposed by Cranenbrouck et al6 was used. 100 propagules of the strain were used, which were placed on a membrane (0.44 μm pore) and washed three times with sterile distilled water. Subsequently, they were contacted with a 2 % Chloramine T solution and two drops of Tween 20 for 10 minutes. They were then washed three times with sterile distilled water and treated with a 10-minute antibiotic solution containing streptomycin sulfate (0.02 %) and gentamicin sulfate (0.01 %), which was sterilized with the help of a Milipore filter (type HA, 4.0 cm in diameter and 0.22 μm pore). After this time the membrane with the propagules was transferred to the antibiotic solution, previously filtered in sterile Petri dish (90 mm diameter).
For the control of contaminants, four Petri dishes were randomly placed in the biosafety cabinet with specific means for fungi (Maltose Agar Sabouraud) and for bacteria (Nutrient Agar).
In all cases a Fully Randomized Design was used for the assembly of the trials.
Experiment 1. Evaluation of the percentage of germination and contamination of propagules of Rhizoglomus sp. (INCAM-11) under in vitro conditions
In this experiment, two bioassays were performed with the purpose of evaluating the efficiency of the methodology proposed by Cranenbrouck et al 6 to guarantee the disinfection and subsequent germination of propagules of INCAM-11. Each of the bioassays was repeated twice.
Bioassay 1. In this study the propagules were inoculated in SRM medium, immediately after the end of the disinfection treatment 6. 20 Petri dishes (90 mm diameter) were inoculated, at the rate of 5 propagules per plate, for a total of 100 propagules. At the end of the treatment, the plates were sealed with parafilm (Pechiney, Plastic Packaging, Chicago, IL 60631) and incubated (Binder Incubator, Belgium) in the dark for 60 days at 27 °C.
Bioassay 2. In the second trial two times of exposure to the antibiotic solution were compared with the objective of decreasing the contamination of the propagules and increasing the germination percentages. Two treatments were performed, one in which the propagules were inoculated in SRM medium just after disinfection (AD) and another in which the propagules were kept in the solution for 24 hours after the disinfection treatment (24 AD). Five propagules were also inoculated per plate and 20 plates were used per treatment. At the end of the inoculation the plates were sealed with parafilm and incubated under the same conditions to the previous bioassay.
The plates were examined every four days to assess the germination and contamination percentages of the propagules until the 60th day after disinfection
Determinations made. The determinations corresponding to germination and contamination percentage were made. A germinated propagule was considered when it showed growth of the germination tube with a length greater than the diameter of the spore, either from the sporophore or from the end of the support hyphae 7.
In the case of contamination, the number of contaminated propagules on a plate was considered with respect to the total number of propagules planted in each.
In the trial comparing the exposure time to the antibiotic solution, the percentages of increase and decrease in germination and contamination were calculated, respectively.
Experiment 2. Evaluation of germ tube growth
This experiment was designed with the purpose of performing a dynamics of propagation germ tube of propagules of INCAM-11. The variable began to be evaluated from the moment in which germination tube growth was observed from the sporophore or the support hyphae. The measurements were made every four days with a micrometer coupled to the dissecting microscope (Novel, 40X magnification), starting from the beginning of the new hypha formed and to the apex of it. This study was extended until it was observed that the hyphae stopped their growth.
This study also compared the length of the germinative tube of propagules to which both disinfection treatments described in bioassay 2 of experiment 1 were applied.
Statistical analysis
After checking the normality and homogeneity of variance (Brown-Forsythe Test), using the statistical package SPSS Statistics 19 (IBM), the data were submitted to Variance Analysis of simple classification and subsequent Tuckey Test, in order to identify significant differences between treatments ap <0.05.
The determinations corresponding to germination and contamination percentage were made. A germinated propagule was considered when it showed growth of the germination tube with a length greater than the diameter of the spore, either from the sporophore or from the end of the support hyphae 7.
In the case of contamination, the number of contaminated propagules on a plate was considered with respect to the total number of propagules planted in each.
In the trial comparing the exposure time to the antibiotic solution, the percentages of increase and decrease in germination and contamination were calculated, respectively.
RESULTS AND DISCUSSION
Experiment 1. Evaluation of the percentage of germination and contamination of propagules of Rhizoglomus sp. (INCAM-11) under in vitro conditions
The disinfection of mycorrhizal fungal spores used as inoculum is crucial for the formation and successful development of mycorrhization in in vitro conditions, since the presence of contaminants is one of the biggest problems that undermines the study of these microorganisms under controlled conditions; In addition, disinfection methodologies must be established for each species of fungus in particular as the effect of disinfectants or their combinations vary depending on the species used 8.
Bioassay 1. In this trial it was found that the disinfected propagules of INCAM-11 began to germinate between the sixth and eighth day after treatment and continued to germinate progressively until day 28. This behavior corresponds to that proposed by other authors 9 , who reported that AMF propagules take a germination 2 to 30 days after the end of the disinfection treatment.
The average germination values, at 60 days, were less than 50 %, as can be seen in Figure 1, while 60 % of the inoculated plates showed some growth of microbial contaminants associated with the propagules (Figure 2).
Apparently, the contaminants that proliferated in the plates were associated with the propagules before disinfection (Figure 2), since in the control plates containing specific means for fungi (Maltose Agar Sabouraud) and bacteria (Nutrient Agar), it was not observed microbial growth, which shows that the contamination of the propagules came from their walls.
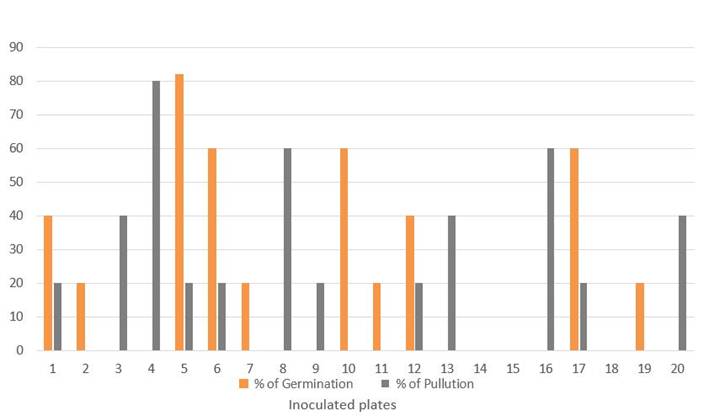 The bars represent the means of five propagules
The bars represent the means of five propagulesFigure 1 Behavior of the estimated Germination and Contamination percentages in each of the Petri dishes inoculated with propagules of INCAM-11 in Bioassay 1
The efficiency of the disinfection methodology used has been proven in numerous previous studies 2,8,10 and in a certain number of AMF strains that are now part of the two in vitro collections of AMF in the world (GINCO Belgium and GINCO Canada). However, in the case of this particular strain, the concentrations of contaminants that proliferated after the disinfection treatment are high, with percentages ranging between 20 and 80 in each of the plates (Figure 1).
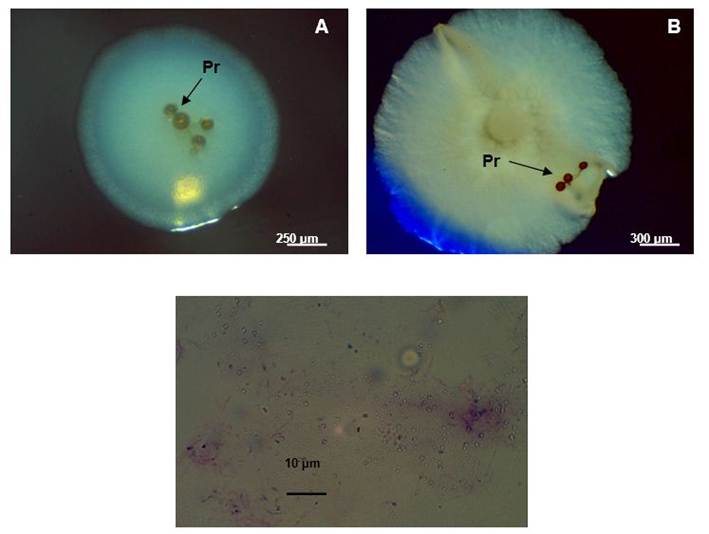 Photos taken under the dissection microscope (A and B) (Novel, 40X). Photo C taken under an optical microscope (Novel, 1000X)
Photos taken under the dissection microscope (A and B) (Novel, 40X). Photo C taken under an optical microscope (Novel, 1000X)Figure 2 Images of bacterial contamination associated with disinfected propagules (Pr) of INCAM-11 (A and B). Gram stain of the most frequent contaminant (C)
The germination values reached are generally similar to those found by other authors 11, who reported, in spores of Gigaspora margarita (Becker & Hall) and Scutellospora heterogama (Nicolson & Gerdemann - Walker & Sander) inoculated in Agar Agua medium , values close to 60 % in the presence of Chloramine T (2 %) and exposure times to disinfectants + antibiotic between 3 and 6 minutes. However, some authors 8, applying the same disinfection methodology as used in this study and also using the SRM culture medium, reported only 33 % germination in spores of Rhizoglomus sp.
Germination of AMF propagules can occur in the absence of signals derived from the host, although they cannot complete their life cycle under these conditions. However, there are some factors that can stimulate germination and even inhibit it, such as the composition of the culture medium, pH, some organic compounds, moisture and associated microorganisms 12.
In the specific case of microorganisms, there are certain microbial groups that can, not only stimulate the germination of the propagules, but also inhibit it and live, usually, associated with the spores and hyphae of AMF, either in the cell lumen , or attached to its walls. They may also have a share in the benefits that these fungi report to plants 13.
The investigations, using totally disinfected spores of Funneliformis mosseae allowed to isolate a large number of microorganisms that grew inside the spores 14. These authors managed to subculture 11 isolates belonging to several microbial groups that were not only able to stimulate spore germination and hyphae branching, but also solubilized phosphate and fixed atmospheric nitrogen.
On the other hand, the disinfection studies in Rhizoglomus clarus spores allowed to demonstrate that some contaminants that grew around the spores hindered the emergence and growth of the germ tube, so that their germination was limited 15.
However, it is important to highlight that there are certain groups of microorganisms not considered contaminants that inhabit the walls of the spores and that play a beneficial role on the germination and the length of the germination tube 12, so that the use of methodologies of non-aggressive disinfection, which does not completely eliminate the microorganisms that cohabit with the spores, is the key to guarantee germination success 14.
Some microorganisms of the genus Pseudomonas and Corynebacterium, can increase the germination of spores of Rhizoglomus versiforme and directly affect the mechanisms of destruction of the external wall, exchange molecular material and combine some final products of the synthesis of each of the microorganisms involved 15.
In this regard, in another investigation, a marked stimulation of the germination of funneliformis mosseae spores was reported when they were inoculated in the presence of microbial species previously isolated from their interior 14.
Bioassay 2. With the objective of reducing the contamination of the propagules and increasing the germination percentages, this test was carried out, extending the exposure time to the antibiotic solution to 24 hours, after the end of the disinfection treatment.
Figure 3 represents the effect of this variation on the behavior of both variables.
Regarding germination, it was observed that the propagules that were exposed to the antibiotic solution for 24 hours prior to inoculation began to germinate two to four days before the previous bioassay, that is, at four days, and the percentages Germination gradually increased in relation to the propagules that did not receive this treatment, reaching a 75 % increase. Likewise, prolonged exposure to the antibiotic solution contributed to reducing contamination levels by 46 %.
Many studies have been carried out with spores of different AMF genera under in vitro conditions where the implementation of disinfectant agents such as Chloramine T, Tween 20 and antibiotic solutions (eg Streptomycin, Gentamicin Sulfate, Cephalexin, etc.) has been successful. different concentrations and exposure times 2,5,8; however, keeping the propagules for 24 hours exposed to the antibiotic solution had not been reported so far.
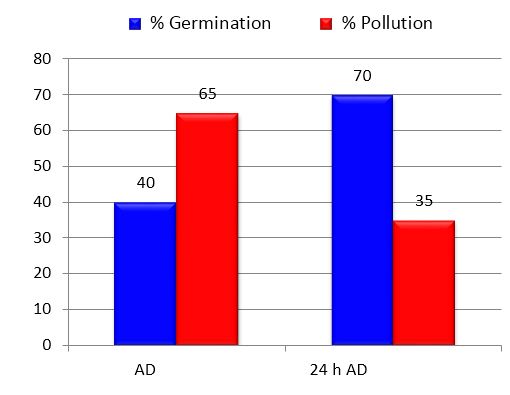 Bars of the same color with different letters differ from each other. The bars are the averages of 20 plates containing five propagules each. Significant differences for p <0.05 according to Tukey's test. - AD after disinfection; 24 h DD - 24 hours after disinfection
Bars of the same color with different letters differ from each other. The bars are the averages of 20 plates containing five propagules each. Significant differences for p <0.05 according to Tukey's test. - AD after disinfection; 24 h DD - 24 hours after disinfectionFigure 3 Effect of the residence time in the antibiotic solution before inoculation on the percentages of germination and contamination of propagules of INCAM-11 in the bioassay 2
The aforementioned confirms that in order to obtain satisfactory results in the disinfection of spores, the concentrations of disinfectants and antibiotics, as well as the duration of the treatments should be adapted depending on the levels of contamination and the sensitivity of the spores.
Although contamination was significantly reduced in this bioassay in relation to bioassay 1, the levels obtained are considered high when compared to those reported by Danesh et al 5, using similar methodology. These authors obtained contamination percentages of less than 5 % in treated spores of Rhizoglomus intraradices.
The concentrations of microorganisms in the AMF propagules must be related to the fungal species and the substrate on which they develop before sterilization. During the colonization process, AMF interacts with numerous bacteria and spores and hyphae provide sites where certain microbial populations commonly inhabit 12, so that both criteria are conditions for their propagules to live with more or less number of microorganisms
The results of this first experiment demonstrate that by modifying existing methodologies the expected effects can be obtained. So that if INCAM-11 spores are kept in antibiotic solution for 24 hours, using the methodology of Cranenbrouck et al6, before their inoculation in SRM culture medium, their germination percentages are increased by 75 %, pollution levels are reduced by 46 % and the occurrence of the germination process is advanced from two to four days.
The presimbiotic growth of AMF is a decisive phase for the survival of these fungi since it depends on them that they can find a compatible host to complete their life cycle and the use of in vitro culture techniques has allowed us to study how different factors can , synergistically or individually, affect the growth of the fungus during this early and sensitive stage of its development.
Experiment 2. Evaluation of germ tube growth
In this experiment, an evaluation of the dynamics of growth of the germ tubes of INCAM-11, previously disinfected, was carried out using both disinfection treatments (Bioassay 2) to determine the moment at which they stop their growth in axenic conditions.
As can be seen in Figures 4 and 5, the propagules begin to germinate as well as in the previous tests, between days four and eight after disinfection, depending on the treatment. From this moment on, the germination tubes continue to grow exponentially until they reach constant values around day 56 (Figure 4).
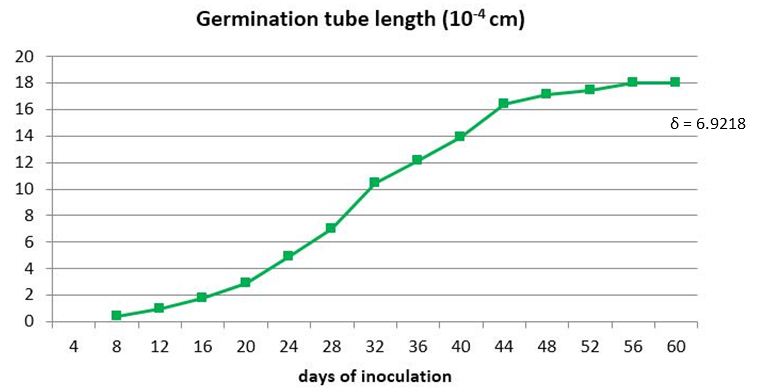 The points correspond to the average of 100 propagules
The points correspond to the average of 100 propagulesFigure 4 Dynamics of the germ tube growth of INCAM-11 propagules inoculated to germinate in SRM medium for 60 days
When the propagules remain in the antibiotic solution for 24 hours, they begin to germinate earlier and higher values of germ tube length (LTG) are reached, showing, from day 16, significant differences between treatments (Figure 5) and percentages of 35 % increase on average.
The germination of the propagules is not homogeneous, but varies on the day of onset, the length reached and the moment they stop their growth.
In this experiment, the germinating hyphae that grew for more days stopped their growth on day 56 (Figures 4 and 5), regardless of when they had begun to germinate, estimating an average of 30 days during which the hyphae continued to grow in search of signals from a potential host before stopping their progression, probably due to depletion of the propagule reserves.
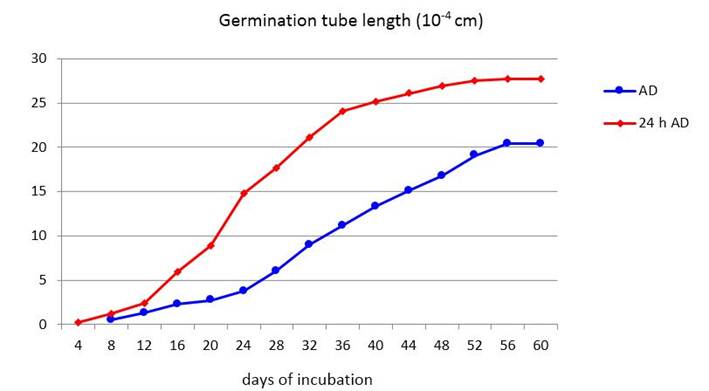 The points correspond to the average of 100 propagules. Significant differences for p <0.05 according to Tukey's test. AD - after disinfection; 24 h AD - 24 hours after disinfection
The points correspond to the average of 100 propagules. Significant differences for p <0.05 according to Tukey's test. AD - after disinfection; 24 h AD - 24 hours after disinfectionFigure 5 Effect of the time of exposure to the antibiotic solution on the growth of the propagules germinative tube of INCAM-11
Several authors assure that the growth of the germ tube is dependent on the availability and quantity of spore reserves 16 and that the protoplasm contains all the organelles necessary to ensure its subsequent development. This consists in the straight growth of the running hypha in search of a potential host and the successive thinner branches that occur to explore the surrounding environment. In the event that there is no contact with any root or that any signal from a host is not detected, the germination tube stops its growth, the protoplasm retracts from the apex of the hypha and is sequestered, emptying the hypha, through the production of successive septa.
Other authors have found similar results in relation to the time it takes for germ tubes of AMF spores to stop their growth 17. According to these authors, in a germination experiment of previously disinfected R. intraradices spores, they observed the formation of septa at the apical end of the hypha, the retraction of the cytoplasm and the cessation of hyphal growth, between two and five weeks after their inoculation in culture medium and in the absence of transformed roots.
Apparently there is no high relationship between the moment at which the propagules germinate and the time that elapses until the germinating hyphae stop growing, which could be confirmed with the correlations made (r = 0.424 at p ≤ 0.05).
The length of the germination tubes and the germination of the spores are parameters that are not related, therefore a higher germination rate does not imply that the germination tubes are greater 18. The length that these structures can reach is more related to the size of the spores and the reserves they can store 18 and is manifested both in spores of different genera 12, and in individuals of the same species 18.
CONCLUSIONS
The residence time of the spores in antibiotic solution for 24 hours before inoculation in SRM culture medium, increases the germination percentages, decreases the pollution percentages and germination is advanced from two to four days. So far, no previous study had taken into account this modification, so the use of it in disinfection methodologies will favor the success of in vitro cultures.
The germination hyphae of Rhizoglomus sp. (INCAM - 11) they spread in the culture medium for approximately 30 days independently, before stopping their growth, which contributes to the subsequent establishment of the association with transformed roots.
BIBLIOGRAFÍA
1. IJdo M, Cranenbrouck S, Declerck S. Methods for large-scale production of AM fungi: past, present, and future. Mycorrhiza. 2011;21(1):1-16. doi:10.1007/s00572-010-0337-z [ Links ]
2. Srinivasan M, Kumar K, Kumutha K, Marimuthu P. Establishing monoxenic culture of arbuscular mycorrhizal ,fungus Glomus intraradices through root organ culture. Journal of Applied and Natural Science. 2014;6(1):290-3. doi:10.31018/jans.v6i1.417 [ Links ]
3. Fernández Suárez K, Declerck S, Fernández García L, Ortega Delgado E. Aplicación del sistema de Planta Donante de Micelio (PDM) en la micorrización in vitro de papa. Cultivos Tropicales. 2017;38(1):31-8. [ Links ]
4. Fernández Suárez K. Los sistemas de cultivo in vitro aplicados al estudio de los hongos micorrízicos arbusculares (HMA). Cultivos Tropicales. 2012;33(2):33-43. [ Links ]
5. Danesh YR, Najafi S, Demir S. Using In Vitro Culturing Technique for Studying Life Cycle of Arbuscular Mycorrhizal Fungus (AMF) Glomus intraradices. YYU JAGR SCI. J AGR SCI. 2016;26(2):161-7. [ Links ]
6. Cranenbrouck S, Voets L, Bivort C, Renard L, Strullu D-G, Declerck S. Methodologies for in Vitro Cultivation of Arbuscular Mycorrhizal Fungi with Root Organs. In: In Vitro Culture of Mycorrhizas [Internet]. Springer, Berlin, Heidelberg: Soil Biology; 2005 [cited 10/05/2019]. p. 341-75. doi:10.1007/3-540-27331-X_18 [ Links ]
7. Fernández Suárez K, Pérez Ortega E, García M, R L. La kinetina ribósido como estimulador de la germinación In Vitro de esporas de Glomus clarum. Cultivos Tropicales. 2015;36(3):45-9. [ Links ]
8. Pérez UA, Ramírez MM, Moreno LM, Franco M. Metodología para la desinfección y germinación de esporas y fragmentos de raíces micorrizados con Glomus sp. (GEV02) para su uso bajo condiciones in vitro. Revista Corpoica. 2011;12(2):143-50. [ Links ]
9. Cranenbrouck S, Declerck S. Spore and root association. CESAMM Team. 2016. [ Links ]
10. Fernández K, Fernández F, Rivera R, Olalde V. Metodología para la germinación de esporas de Glomus mosseae. Cultivos Tropicales. 2005;26(2):11-6. [ Links ]
11. Filho AC, Siqueira JO, de Oliveira E. Desinfestação superficial de esporos de fungos micorrízicos vesicular-arbusculares. I. Efeitos da concentração e tempo de exposição de agentes desinfestantes e antibióticos. Pesquisa Agropecuária Brasileira. 1994;29(7):1119-27. [ Links ]
12. Cruz AF, Ishii T. Arbuscular mycorrhizal fungal spores host bacteria that affect nutrient biodynamics and biocontrol of soil-borne plant pathogens. Biology Open. 2012;1(1):52-7. doi:10.1242/bio.2011014 [ Links ]
13. Jansa J, Bukovská P, Gryndler M. Mycorrhizal hyphae as ecological niche for highly specialized hypersymbionts - or just soil free-riders? Frontiers in Plant Science. 2013;4:1-8. doi:10.3389/fpls.2013.00134 [ Links ]
14. Mirabal L de los A, Ortega E. Comunidad microbiana asociada a los Hongos Micorrizógenos Arbusculares [Tesis de Doctorado]. [La Habana]: Universidad de la Habana. Facultad de Biología; 2011. [ Links ]
15. Karthikeyan B, Abitha B, Henry AJ, Sa T, Melvin, M. Interaction of Rhizobacteria with Arbuscular Mycorrhizal Fungi (AMF) and Their Role in Stress Abetment in Agriculture. In: Recent Advances on Mycorrhizal Fungi [Internet]. Suiza: Springer International Publishing; 2016 [cited 10/05/2019]. p. 120-3. doi:10.1007/978-3-319-24355-9_11 [ Links ]
16. Bago B, Cano C. Breaking Myths on Arbuscular Mycorrhizas in Vitro Biology. In: Declerck S, Fortin JA, Strullu D-G, editors. In Vitro Culture of Mycorrhizas [Internet]. Berlin, Heidelberg: Springer: Soil Biology; 2005 [cited 10/05/2019]. p. 111-38. doi:10.1007/3-540-27331-X_7 [ Links ]
17. Hildebrandt U, Janetta K, Bothe H. Towards Growth of Arbuscular Mycorrhizal Fungi Independent of a Plant Host. Applied and Environmental Microbiology. 2002;68(4):1919-24. doi:10.1128/AEM.68.4.1919-1924.2002 [ Links ]
18. Maia LC, Yano-Melo AM. Germination and germ tube growth of the arbuscular mycorrhizal fungi Gigaspora albida in different substrates. Brazilian Journal of Microbiology. 2001;32(4):281-5. doi:10.1590/S1517-83822001000400005 [ Links ]
Received: September 19, 2018; Accepted: May 16, 2019











 texto en
texto en 


