Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Hematología, Inmunología y Hemoterapia
versión On-line ISSN 1561-2996
Rev Cubana Hematol Inmunol Hemoter v.26 n.4 Ciudad de la Habana oct.-dic. 2010
PRESENTACIÓN DE CASOS
Cell therapy for the treatment of lower limb lymphedema. Case report
Terapia celular en el tratamiento de linfedema de miembros inferiores. Presentación de un caso
Dr. Pedro Goicoechea-DíazI; Prof. DrC. Porfirio Hernández-RamírezII; Dr. Heriberto Artaza-SanzI; Dr. Lázaro Cortina-RosalesII; Dra. Vianed Marsán-SuárezII; Dr. Yamilé Peña-QuíanIII; Dr. Alejandro Perera-PintadoIII
I Hospital General Docente "Enrique Cabrera". Ciudad de La Habana, Cuba.
II Instituto de Hematología e Inmunología. Ciudad de La Habana, Cuba.
III Centro de Investigaciones Clínicas. Ciudad de La Habana, Cuba.
ABSTRACT
Although lymphedema is a common disabling disease causing significant morbidity for affected patients, treatment for this condition remains limited and largely ineffective. Some reported data suggest that some bone-marrow derived cells may play a role in lymphangiogenesis. It appears that blood vessels and lymphatic vessels might use the same population of cells for vasculogenesis and lymphangiogenesis. Therefore, adult stem cell therapy could be a new useful strategy for the treatment of lymphedema. We report a resolution of a severe lower limb bilateral lymphedema after implantation of autologous adult stem cells derived from bone marrow. As far as we know, this is the first reported case with chronic lower limb lymphedema treated successfully with autologous cell therapy. This procedure is a low-cost, relatively simple and easy to perform option that opens new ways for the treatment of lymphedema.
Key words: Stem cell, lymphedema, bone-marrow derived cells, lymphangiogenesis.
RESUMEN
Aunque el linfedema es una enfermedad crónica inhabilitante común que causa morbilidad significativa en los pacientes afectados, el tratamiento para esta enfermedad se mantiene muy limitada y en la mayor parte de los casos resulta ineficaz. Algunos datos reportados sugieren que algunas de las células madre derivadas de la medula ósea pueden intervenir en la linfangiogénesis. Al parecer, los vasos sanguíneos y los vasos linfáticos podrían usar la misma población celular para la vasculogénesis y la linfangiogénesis. Por consiguiente, la terapia con células madre adultas podría ser una nueva estrategia útil para el tratamiento de linfedema. En el presente trabajo se informa la resolución de un linfedema bilateral severo de miembros inferiores después de la implantación de células madre autólogas derivadas de la médula ósea. Hasta donde sabemos, este es el primer caso de linfedema crónico de los miembros inferiores tratado exitosamente con células madre autólogas. Este método de tratamiento es económico, relativamente simple, fácil de realizar y una opción que abre nuevas vías para el tratamiento del linfedema.
Palabras clave: células madre, linfedema, células derivadas de la médula ósea, linfangiogénesis.
INTRODUCTION
Lymphedema is a chronic condition characterized by the abnormal accumulation of interstitial fluid due to insufficiency of the lymphatic system, either as a primary or as a secondary disorder. Although it is a common disabling disease causing significant morbidity for affected patients, treatment for this condition remains limited and largely ineffective.1
Recently stem-cell based therapy has been the focus of attention for inducing therapeutic angiogenesis and the therapeutic potential of adult stem cells in the treatment of peripheral arterial disease has become increasingly evident during the last years.2
Some reported data suggest that some bone-marrow derived cells may play a role in lymphangiogenesis.3,4 It appears that blood vessels and lymphatic vessels might use the same population of cells for vasculogenesis and lymphangiogenesis.5 Therefore, adult stem cell therapy could be a new useful strategy for the treatment of lymphedema.
Here we report a resolution of a severe lower limb bilateral lymphedema after implantation of autologous adult stem cells derived from bone marrow.
CASE REPORT
A 58 -year -old man was referred to our service of angiology with severe swelling of both lower extremities. This swelling started two years earlier after recurrent episodes of lymphangitis. The diagnosis of lymphedema of the inflammatory type was performed and conservative therapy was initiated, including compression bandaging. Despite this treatment the swelling became progressively more severe. This condition limited his walking in a marked degree and disabled him to go upstairs.
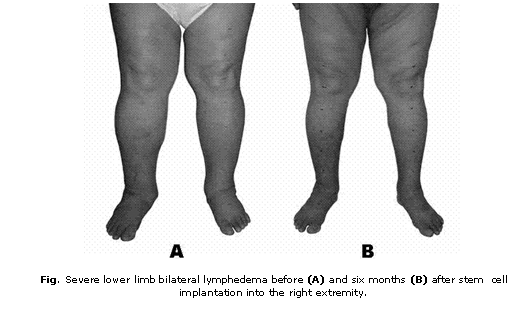
Except the above mentioned lymphangitis episodes, the patient did not have a noteworthy past medical history. Physical examination revealed a body weight of 137 kg, a blood pressure of 120/80 mm Hg and severe lower limb bilateral lymphedema affecting both extremities up to the inguinal regions (fig. 1A). No other pathological findings were revealed. The circumferences of his lower extremities were measured 7 cm above the knee, at the knee, 7cm below the knee, at the ankles and at the metatarsus (table).
Laboratory findings, including a complete blood count, sedimentation rate, urinalysis findings, liver function tests and renal tests were unremarkable. A chest radiograph and an electrocardiogram showed normal results. A 99mTc sulphide colloidal lymphoscintigraphy6 showed absence of flow at both lower extremities.
As patient was considered unresponsive to conservative therapy, taking into account worsening of swelling in both extremities as well as serious worsening in quality of life, an autologous transplantation of Granulocyte Colony-Stimulating Factor (G-CSF) mobilized peripheral blood stem cell (PBSC) in the most affected extremity was proposed. Scientific and Ethics Committees of participating institutions approved this treatment and the patient gave written informed consent.
For bone marrow mononuclear cell mobilization, the patient was previously subcutaneously injected with human recombinant G-CSF (Leuko CIM, CIMAB SA, La Habana, Cuba). The whole procedure, including peripheral blood collection and mononuclear cell (MNC) concentration adding hidroxyetilstarch (HES) 6 % to the collected blood, was performed as previously described.7 A final volume of 140 mL of concentrated cell suspension was obtained. Absolute MNC count was 8,4 x 109 and CD34+ absolute stem cell number was 42 x 106. Cell viability was 95 %.
Under propofol 1 %, sedation and in sterile conditions concentrated cells were implanted in the most affected extremity (right limb) by multiple circumferential injections into the leg and distal half of the thigh. In addition, cells were also injected into the dorsum of the foot. A volume of 0,75 mL of the cell suspension was implanted 1-1,5 cm deep into each injection site with a 3 x 3 cm grid, using a 24 - gauge needle. Total injection volume was 120 mL.
Slight leakage of fluid from the tissues observed at the injection sites, disappeared 48 hours after cell implantation. During this period, compression bandaging was used and there after an elastic stocking was indicated. No other related side- effect was observed throughout the therapeutic procedure or within the whole follow-up period.
One week after cell implantation a mild improvement of foot swelling was appreciated at the treated extremity. Subsequently, progressive improvement of lymphedema was observed and striking improvement of bilateral lymphedema was obtained with 19 kg body weight lost during the 6 months following cell implantation (fig. 1B). Six months after treatment the patient showed reduced circumferences of both extremities (table). Follow-up lymphoscintigraphy was not performed because patient did not accept this study. Improvement was sustained during one and a half year follow-up.
DISCUSSION
In recent years, much attention has been given to lymphangiogenesis and new advances in the study of lymphedema have been obtained.8
Lymphatic vessels and blood vessels are essential collaborating parts of the circulatory system. The lymphatic vessels differ in many ways from the blood vessels, but they also share many properties. Recently, new information about the regulation of lymphangiogenesis has been gained, and the factors known to regulate blood vessels have been shown to be involved in the biology of the lymphatic vessels.9 The development of blood and lymphatic vascular systems is primarily regulated by vascular endothelial growth factor (VEGF) family members: VEGF-A, VEGF-B, VEGF-C, VEGF-D and placenta growth factor.10 It is considered that VEGF-A is the most important of them for the control of angiogenesis, whereas VEGF-C and VEGF-D are the main factors that control lymphangiogenesis.
From the clinical point, various therapeutic procedures have been proposed for selected patients with lymphedema, who are unresponsive to conservative therapy.8,9 Several surgical options with promising results have been reported, including lymphatic microsurgery, autologous lymphatic tissue implants and circumferential suction - assisted lipectomy.8,11-13
More recently, based on novel findings on the molecular mechanisms involved in lymphangiogenesis, encouraging results have been obtained with VEGF-C gene therapy and with human recombinant VEGF-C in experimental animal models, which provide attractive procedures for pro-lymphoangiogenic therapy in lymphedema.14,15 Known similarities between the regulation of blood and lymphatic vessels, and the preclinical and clinical studies that have provided evidence that implantation of bone marrow derived cells into ischemic limbs can improve tissue vascularization, encouraged us to use cell therapy for the treatment of lymphedema.
Following autologous mobilized PBSC implantation, our case showed an astonishing clinical recovery with marked improvement of bilateral lymphedema, despite the fact that cells were implanted in only one of the affected extremities.
Up today, the mechanisms through which the transplanted cells might improve tissue recovery remain unknown. Several hypothesis have been suggested including transdifferentiation, cell fusion, a paracrine effect by release of various cytokines and growth factors or maybe an addition of more than one mechanism.5,16,17 It has been referred that adult human progenitor cells from bone marrow are potent sources of VEGF.17,18 On this point, it is important to underline that VEGF-C and VEGF-D are specific regulators of lymphangiogenesis.10
It is accepted that hematopoietic cells may release different growth factors and cytokines and a fraction of CD34+ cells may acts as lymphatic / vascular endothelial precursors cells.2,3
There are at least two possibilities that might explain why G-CSF mobilization plus the transplantation into local affected tissues of the collected and concentrated PBSCs can result in the excellent therapeutic improvement obtained. The first is that local injections of G-CSF mobilized PBSCs into the selected lower extremity directly bring a number of circulating endothelial precursor cells into affected tissues where these cells can initiate lymphangiogenesis. The second is that a large number of transplanted cells can secrete in vivo in the injected sites several cytokines and grown factors known to stimulate lymphangiogenesis and that may produce a paracrine effect in a similar way as has been suggested in ischemic diseases.17,19 Another possible related mechanism is based on the in vivo (endogenous) availability of a pool of systemic and circulating G-CSF mobilized PBSCs that might be recruited to the affected tissues, contributing in this way to lymphangiogenesis. Some of these possibilities may coexist in this therapeutic approach.
By the other side, we were surprised by the fact that the contralateral, also affected but non-treated, lower limb, also showed a notable improvement. Although we have not a proved explanation for the mechanisms involved in this therapy related effect, it could be suggested that endogenous mobilized PBSCs were recruited to the affected tissues in this limb, as aforementioned, and in this way they could in principle act similarly to the local exogenous transplanted cells facilitating the recovery of the swollen tissues inducing certain degree of lymphangiogenesis. As a pure speculation, this observation raises also the possibility that the classical inter cellular coordinative communicating system may be more complex than previously thought.
In some cases increased serum values of cytokines and growth factors secreted by the exogenously implanted cells have been detected.20 Perhaps, a telecrine effect of these circulating soluble products might exist in certain cases with an action on distant target-cells; this effect might be in addition to the paracrine effect previously suggested. This possibility would help to explain in part our result and also others not yet completely explained, related to improvement of glucose metabolism in diabetic patients who received mononuclear cell implantation into the lower extremities because of ischemic disorders.21,22
As far as we know, this is the first reported case with chronic lower limb lymphedema treated successfully with autologous cell therapy. This method of treatment is a low-cost, relatively simple and easy to perform option that opens new ways for the treatment of lymphedema. Our observation is supported by the results obtained in a recent controlled study in patients with breast cancer related arm lymphedema.23
However, further studies are needed in order to obtain an accurate evaluation of the efficacy and long term safety of this novel therapeutic strategy in patients with lymphedema.
REFERENCES
1. Yoon YS, Marayama T, Gravereux E, Tkebuchava T, Silver M, Curry C, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 2003;111:717-25.
2. Hernández P, Cortina L, Artaza H, Pol N, Lam RM, Dorticós E, et al. Autologous bone-marrow mononuclear cell implantation in patients with severe lower limb ischaemia: A comparison of using blood cell separator and Ficoll density gradient centrifugation. Atherosclerosis 2007;194:e52-6.
3. Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 2003;9:702-12.
4. Salven P, Mustjoki S, Alitalo R, Alitalo K, Raffi S. VEGFR-3 and CD 133 identify a population of CD 34+ lymphatic/vascular endothelial precursor cells. Blood 2003;101:168-72.
5. Religa P, Cao R, Biorndahl M, Zhou Z, Zhu Z, Cao Y, et al. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood 2005;106:4184-90.
6. Lee AF. Lymphoscintigraphy of the extremities. In: O´Connor MK, editor. The Mayo Clinical Manual of Nuclear Medicine. New York: Churchill Livingstone; 1996. pp. 513-9.
7. Hernández P, Artaza H, Díaz AJ, Cortina LD, Lam RM, Pol N, et al. Autotrasplante de células madre adultas en miembros inferiores con isquemia crítica. Experiencia en Cuba. Rev Esp Invest Quirúrg 2007;10:204-11.
8. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: A comprehensive review. Ann Plast Surg 2007;59:464-72.
9. Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev 2002;8:673-700.
10. Sato Y. VEGFR1 for lymphangiogenesis arterioscler. Tromb Vasc Biol 2008;28:604-5.
11. Campisi D, Davini D, Bellini C, Taddei G, Villa G, Fulcheri E, et al. Lymphatic microsurgery for the treatment of lymphedema. Microsurgery 2006;26:65-9.
12. Campisi C, Da Rin E, Bellini C, Bonioli E, Boccardo F. Pediatric lymphedema and correlated syndromes: Rle of microsurgery. Microsurgery 2008;28:138-42.
13. Belcaro G, Errichi BM, Cesarone MR, Ippolito E, Dugall M, Ledda A, et al. Lymphatic tissue transplant in lymphedema a minimally invasive, out patient, surgical method: a 10-year follow-up pilot study. Angiology 2008;59:77-83.
14. Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2, VEGF-A, and VEGF-C induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res 2004;95:664-70.
15. Lohela M, Saaristo A, Veikkola T, Alitalo K. Lymphangiogenic growth factors, receptors and therapies. Thromb Haemost 2003;90:167-84.
16. Körbling M, de Lima MJ, Thomas E, Khanna A, Najjar AM, Gu J, et al. Fusion of circulating blood cells with solid organ tissue cells in clinical stem cell transplant: A potential therapeutic model? Reg Med 2008;3:157-64.
17. Méndez-Otero R, de Freitas GR, Andre C, Furtado de Mendoça ML, Friedrich M, Oliveira-Filho J. Potential roles of bone marrow stem cells in stroke therapy. Regen Med 2007;2:417-23.
18. Wang M, Crisotomo PR, Herring C, Meldrum KK, Meldrum DR. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol 2006;291:880-4.
19. De Araujo JD, de Araujo Filho JD, Ciorlin E, Ruiz MA, Ruiz LP, Greco OT, et al. A terapia celular no tratamento da isquemia crítica dos miembros inferiores. J Vas Bras 2005;4:357-65.
20. Tachi Y, Fukui D, Wada Y, Koshikawa M, Shimodaira S, Ikeda U, et al. Changes in angiogenesis related factors in serum following autologous bone marrow cell implantation for severe limb ischemia. Exp Opin Biol Ther 2008;8:705-12.
21. Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor - mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 2005;28:2155-60.
22. Novoa E, Medina A. Therapeutic angiogenesis in arterial ischaemic limbs by autologous bone marrow transplantation (ABMT). The Conzi´s effect in human diabetes mellitus. Arch Med Int (Uruguay) 2007;29(Suppl 1):S24-S25.
23. Hou C, Wu X, Jin X. Autologous bone marrow stromal cells transplantation for the treatment of secondary arm lymphedema: A prospective controlled study in patients with breast cancer related lymphedema. Jpn J Clin Oncol 2008;38:670-4.
Recibido: 8 de junio del 2010.
Aprobado: 25 de junio dle 2010.
Prof. DrC. Porfirio Hernández Ramírez. Instituto de Hematología e Inmunología. Apartado 8070, CP 10800, Ciudad de La Habana, Cuba. Tel (537) 643 8695, 8268. Fax (537) 644 2334. E-mail: ihidir@hemato.sld.cu