Introduction
Equine influenza (EI) is still one of the most common highly contagious respiratory diseases affecting equines caused by two equine influenza A virus (EIV) subtypes, H7N7 and H3N8 (formerly known as equi-1 and equi-2 respectively), members of genus Alfainfluenzavirus within the family Orthomyxoviridae. EIV causes high morbidity (outbreaks) but low mortality; H3N8 is the parent subtype of all present EIV strains.1
In susceptible equines, clinical signs of EI include pyrexia, nasal discharge and a harsh dry cough, while pneumonia in young foals and donkeys, and encephalitis in horses, have been described as rare events.2
In Egypt, three outbreaks of EIV H3N8 were recorded in horses, mules and donkeys. The first one occurred in October 1989 in Monufia Governorate in the Nile Delta;3 it is believed to be caused by EIV H7N7 or H7N7 mixed with H3N8.4).The second outbreak in Egypt was reported in 2000 in the Nile Delta and Upper Egypt,5,6 while the most recent and severe EIV H3N8 outbreak was in 2008.7 The infections spread to several provinces in a short time, and the virus had 98% genetic identity with H3N8 viruses from USA and Japan.4
Vaccination is still the best method to overcome and prevent the EIV outbreaks. Currently, there are three different types of EI vaccines: whole inactivated, live attenuated and vaccines based on viral vectors. The whole inactivated EIV vaccine is still the most widely used.8,9,10 In addition, it is well accepted and recognized that emergency vaccination has contributed to reduction of EIV transmission in many countries, both in terms of speed and distance of transmission.11
In Egypt, EI vaccines must contain at least one of both EIV subtypes, H7N7 and H3N8, especially the current circulating H3N8 strain to obtain a potent vaccine.8 Furthermore, trials for the preparation of EI inactivated vaccines using different adjuvants have been conducted at the Veterinary Serum and Vaccine Research Institute (VSVRI); these vaccines have been evaluated by the Central Laboratory for the Evaluation of Veterinary Biological Products (CLVEB).12,13
Foals are the main animals used for the evaluation of EI vaccines. Other animal models have been used to study influenza viruses including mice, guinea pigs and ferrets which have many advantages like negligible antibody titers to influenza viruses, relative low cost, availability, small size, and easy handling and housing.14
The main disadvantage of the mouse model is the need to use mouse-adapted virus to achieve productive infection and clinically apparent signs of disease, while guinea pigs model has the advantage of efficiently transmitting influenza viruses to others of its species without the need to use guinea pig-adapted virus.14) Ferrets have the disadvantage of relatively limited commercial availability, more complex husbandry requirements and greater expense than mouse and guinea pig models, which can make it difficult to perform experiments with adequate power.14
The present work was carried out to provide an alternative animal model (guinea pigs) to horses for the evaluation of EIV vaccines, in order to save cost, effort and time, in addition to the easier handling of guinea pigs and their negligible EI antibody titers.15
Materials and Methods
Antigen
Inactivated lyophilized EIV H3N8 antigen with hemagglutination (HA) titer 7 log2 was supplied by VSVRI, Abbasia, Cairo to be used in the Hemagglutination Inhibition (HI) test for evaluation of EIV antibody titer in collected sera of vaccinated animals.
Vaccine batches
Five monovalent inactivated EI vaccine batches were used in this study. Three batches were provided by VSVRI, having (A/equine/Egypt(H3N8)/6066-NAMRU3-VSVRI/2008) strain, while the other two batches were imported by Zoeits, having (A/equine-2/Kentucky/97(H3N8)-American Lineage) strain.
Experimental animals
Eighteen native breed foals, 6 months old, were reorganized into six groups (three foals/group). In addition, a total number of 30 guinea pigs, Cavia porcelus, of average weight 700 g/wt was divided into six groups (five animals/group). Both foals and guinea pigs were checked to be seronegative (pre-vaccination) for EIV H3N8 by HI test.2
Ethical approval
All experimental animals in this study were conducted in strict accordance and adherence to the relevant policies regarding animal handling according to international, national, and/or institutional guidelines for the care of animals and were approved by the Research Ethical Committee at the National Research Center, Cairo, Egypt.
Experimental design
Each vaccine batch was injected intramuscularly into foals and guinea pigs of each group using a dose of 2 mL for both animal species as follows: vaccine batches No. 1, 2, 3, 4, 5 were injected in groups 1, 2, 3, 4, 5 of foals, respectively and groups 1, 2, 3, 4, 5 of guinea pigs, respectively. Groups No. 6 of both foals and guinea pigs were kept as control groups. Vaccinated groups (1-5) were injected with a booster dose of vaccine, 4 weeks after the first dose.
Foal blood samples were collected from the jugular vein and guinea pig blood samples, by heart puncture, each week after vaccination until the 7th week to monitor antibody titer. Collected blood samples were allowed to clot and sera were separated by centrifugation. Serum samples from foals and guinea pigs were heat inactivated at 56°C for 30 min to remove nonspecific hemagglutinin.2
Hemagglutination-inhibition (HI) test
HI test2,16 was performed using U-bottom micro-titer plates; hemagglutination (HA) test2 was carried out firstly to appropriately determine 4 HA units of EI H3N8 antigen.
The presence of HI antibodies in serum was tested by HI. Briefly, inactivated sera were twofold serially diluted in Phosphate Buffered Saline (PBS), then 4 HA units of reconstituted H3 antigen were added. Plates were shaken and incubated at room temperature (27°C) for 30 min to allow the reaction to take place; then equal volume of 1% washed chicken red blood cells (RBCs) was added to each well and plates were incubated for 30 min at room temperature (27°C). The test was read by tilting the micro-titer plate at an angle to observe RBCs streaming at the bottom of the well. The HI titer was taken as the highest dilution of serum with complete inhibition of agglutination in the serial dilution.
Commercial ELISA
The commercial ELISA kit (IDEXX Influenza A, REF 99-53101/ Lot No. 8140) supplied by IDEXX was used to determine Influenza A virus antibodies in serum samples. The ELISA test was done according to manufacturer’s instructions.
The presence or absence of antibody to influenza A was determined by the sample to negative (S/N) ratio for each sample.
Results and Discussion
The spread of EIV infection and the severity of disease are mostly reduced by the use of potent inactivated EI vaccines containing epidemiologically relevant virus strains.8,9,10
Inactivated EI vaccines are adjuvanted vaccines containing either inactivated whole viruses or their subunits and provide protection by inducing humoral antibody response to the hemagglutinin protein; multiple doses are required to maintain protective levels of antibody.2
For preparation of inactivated EIV vaccines, vaccinal strains are propagated in specific pathogen free embryonated chicken eggs, then the strains are concentrated and purified before inactivation with agents such as formalin or beta-propiolactone.2)
The efficacy requirements for EI vaccines may vary according to the National Authority, but usually include the assessment of the serological response in horses and virus challenge studies in susceptible horses.2)
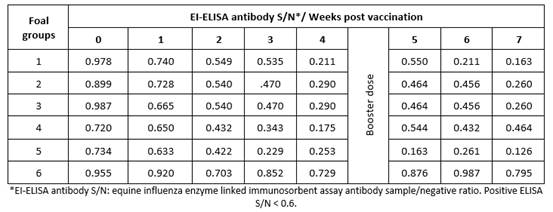
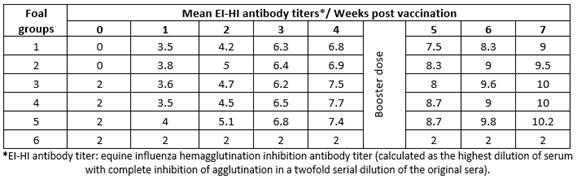
Results obtained by HI test using serum samples from five groups of foals vaccinated with five different lots of EI vaccines revealed that vaccinated animals had EI-specific antibodies recording their highest titers (9 to 10.2) at 7 weeks post vaccination (Table 1); ELISA S/N ratios (0.126 to 0.464) were similar to the HI results (Table 2).
Table 1 Mean hemagglutination inhibition equine influenza antibody titers in sera of vaccinated foals.
Evaluation by ELISA depends on calculation of the signal-to-noise ratio (S/N), which quantifies and compares the level of a desired signal to the level of background noise (undesired signal). S/N was calculated by dividing the mean signal of the clinical study sample by the mean signal of the negative control analyzed, therefore a low S/N ratio indicates that there is a higher than optimal background noise. For the used ELISA kit, S/N values < 0.60 should be considered positive to influenza A antibodies.17
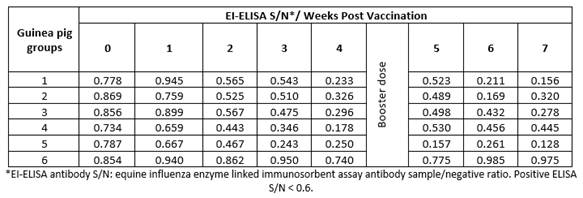
Vaccinated guinea pigs with the same vaccine batches showed the same pattern of HI titers (8.7 - 10, Table 3) and ELISA S/N ratio (0.128 - 0.445, Table 4). These findings indicate the possibility and validity of the use of guinea pigs instead of foals to evaluate EI vaccines.
Table 3 Mean hemagglutination inhibition equine influenza antibody titers in sera of vaccinated guinea pigs.
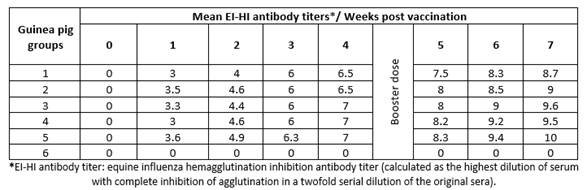
Comparing the HI test results in foals (Table 1) and guinea pigs (Table 3), there was a great relation between them, ranged from 0.985 to 0.999. Also, there was a strong relation between ELISA results in foal (Table 2) and guinea pigs (Table 4), ranged from 0.875 to 0.999.
Regarding the use of laboratory animals as alternative models to horses for the evaluation of EI vaccines, intranasal administration of a single dose of eq/GA/81 ca vaccine virus induced neutralizing antibodies and conferred complete protection against homologous virus challenge in the upper respiratory tract in mice and ferrets.18) In addition, one dose of the eq/GA/81 ca vaccine also induced neutralizing antibodies and conferred complete protection in mice and nearly complete protection in ferrets upon heterologous challenge with the H3N8 (eq/Newmarket/03) virus.2
The level of EI-specific antibodies, measured by HI assays, is correlated to protection against homologous EI strains. Reduced clinical signs of disease and resistance to infection with an EI strain homologous to the vaccine strain have been recorded.19,20