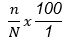Introduction
Highly pathogenic avian influenza (HPAI) H5N8 can infect wild and domestic birds. It has a hemagglutinin (HA) segment of H5 clade 2.3.4.4, which emerged in 2010 in China and other seven segments from multiple avian influenza viruses (AIV). Outbreaks of the HPAI subtype H5N8 virus of clade 2.3.4.4 were firstly reported in poultry farms and wetlands in January 2014 in South Korea.1 Then, the virus was discovered in Siberia, Beringia, and Japan by the summer of 2014. By the end of 2014, the migration of waterfowl played a major factor in the spread of highly pathogenic avian influenza virus (HPAIV) H5N8 throughout East Asia, North America, Africa and Europe.2 This virus has undergone several genetic changes and reassortments, resulting in different subtypes and lineages.3) The most recent wave of H5N8 AIV outbreaks was caused by a novel reassortant virus that originated in Russia and Kazakhstan and disseminated to Europe and Asia.4 In Egypt, The H5N8 AIV strain was first detected in late 2016 in a migratory bird (common coot).4 Phylogenetically, the original virus was closely linked to other H5N8 AIV of clade 2.3.4.4b that were found in Russia in 2016.5 Since then, the H5N8 virus has spread rapidly among different poultry sectors in Egypt, with multiple introduction events and genetic diversity observed among the circulating viruses.6 Six HPAI H5N8 genotypes were identified in Egypt based on the genetic diversity of the internal gene segments. Among them, the most common genotypes found in Egyptian poultry farms in 2019-2021 are G5 and G6.7 According to epidemiologic data, the Egyptian H5N1 virus (clade 2.2.1.2) has been replaced by the HPAI H5N8 virus (clade 2.3.4.4b), which is now the most often identified H5 subtype in Egyptian poultry sectors.8
Vaccines are one of the strategies to prevent and control the spread of this virus among poultry and potentially humans.9 But the evaluation of H5 vaccines against H5N8 worldwide is a challenging task, as the H5N8 virus is constantly evolving and diversifying into different subtypes and lineages.9 Egypt uses commercial AIV vaccines to combat H5 infections, but genetic differences between the recently mutated strains and previously identified Egyptian HPAIV (H5N8) isolated in 2017 have led to ongoing deaths in flocks that received vaccinations from H5N8 infection in various governorates.5,10 In the same context, a study9 mentioned that only two commercial vaccines (Re-5 and Re-6) and an experimental vaccine based on the Egyptian H5N8 strain showed complete protection and reduced virus shedding in chickens; other six commercial vaccines failed to prevent virus shedding and some of them did not protect chickens from mortality. Moreover, another study11 evaluated a new bivalent vaccine (Valley Vac H5Plus NDVg7) that contained the Egyptian H5N8 strain and a Newcastle disease virus (NDV) strain and they found that this vaccine was more effective than the commercial H5+ND7 vaccine in protecting chickens from both H5N8 and NDV infection.
Based on these findings, the most widely used commercial AIV vaccines need to be regularly assessed against newly emerging H5N8 AIV with variable pathogenicity and AIV clinical presentations in order to design the most effective vaccination approach. Consequently, the present study was performed to study the efficacy of some commercial inactivated H5 vaccines from a different lineage against the challenge with HPAI H5N8 virus in broiler chickens, in Egypt.
Materials and Methods
Ethical approval
The study was conducted following the guidelines of the Animal Welfare Committee and protocols were approved by the Research Ethics Committee, Faculty of Veterinary Medicine at Benha University, (Approval number BUFVTM 06-01-23).
Challenge virus and antigen
The clade 2.3.4.4b HPAI H5N8 challenge virus strain (A/chicken/Egypt/ v1526/2020) with accession no. MW600499 was obtained from the National Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute, Giza, Egypt. This virus was propagated in specified pathogen free (SPF) embryonated chicken eggs and the allantoic fluid was harvested. The virus was titrated in SPF embryonated chicken eggs after its purity was verified. To create the viral challenge inoculum, it was diluted in PBS until reached the desired final concentration of 107 median egg infectious dose (EID50)/mL. The virus used in this study became inactive to be used as hemagglutination inhibition (HI) antigen after treating it with 0.05% b-propiolactone for 2 h at 37℃.
Vaccines
Reassortant AIV vaccine (Re 13 & Re 14 strains): oil adjuvant commercial inactivated reassortant avian influenza vaccine prepared from H5N6 subtype, Re-13 strain (A/duck/Fujian/S1424/2020 clade 2.3.4.4h) and H5N8 subtype, Re-14 strain (A/whooper swan/Shanxi/4-1/2020), with manufacture date: 1/2022 and expiry date: 1/2024.
Reassortment AIV vaccine (Re-5 strain): oil adjuvant inactivated reassortant avian influenza vaccine prepared from H5N1 subtype, Re-5 strain (A/duck/Anhui/1/2006 clade 2.3.4), with manufacture date: 1/2022 and expiry date: 1/2024.
Reassortment AIV vaccine (Re-6 &Re-8 strains): oil adjuvant inactivated reassortant avian influenza vaccine prepared from H5N1 subtype, Re-6 strain (A/duck/Guangdong/s1322/10 Clade 2.3.2.1) and H5N1 subtype, Re-8 strain (A/Chicken/Guizhou/4/13 clade 2.3.4.4g), with manufacture date: 3/2022 and expiry date: 3/2024.
Nobilis influenza H5N2 vaccine: oil adjuvant inactivated reassortant avian influenza vaccine prepared from H5N2 subtype, LP strain (A/duck/Potsdam/1402-6/1986), with manufacture date: 3/2022 and expiry date: 3/2024.
MEFLUVAC vaccine: oil adjuvant reassortant avian influenza vaccine prepared from three strains H5N1 clade 2.2.1.1 (2016), H5N1 clade 2.2.1.2 (2017) and H5N8 clade 2.3.4.4b (2018), with manufacture date: 3/2022 and expiry date: 3/2024.
Genetic relatedness between the challenge virus and vaccine seed strains
Avian influenza HA gene nucleotide sequences of the challenge virus and the strains used in each vaccine were obtained from the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov). Next, these sequences were aligned using (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Broiler chickens
A total of 210 day-old (DO) commercial broiler chicks of the Ross breed were kindly provided by El Wadi Company for poultry production. Chicks were housed inside BSL3 chicken isolators during the whole experiment period and were provided drinking water and feed ad libitum.
Vaccination protocol
Two hundred and ten commercial broiler day old (DO) chicks were allocated into seven groups (from Gp1 to Gp7) of 30 chickens each. At 10th day of age, the chickens in Gp1, Gp2, Gp3, Gp4 and Gp5 were vaccinated with inactivated reassortant avian influenza (Re-13 & Re-14 strains) vaccine, inactivated reassortant avian influenza (Re-5 strain) vaccine, inactivated reassortment avian influenza (Re-6 & Re-8 strains) vaccine, Nobilis avian influenza (H5N2) vaccine and MEFLUVAC avian influenza vaccine, respectively. All vaccinations were performed by subcutaneous injection route at the base of the neck (0.5 mL/ chicken). The chickens in Gp6 served as challenged non-vaccinated control group (control positive group) and the chickens in Gp7 were considered as non-vaccinated, non-challenged group (control negative group).
Challenge protocol
At 31st day of age, challenge test was conducted on 20 chickens from each vaccinated group (Gp1, Gp2, Gp3, Gp4 and Gp5), and in the Gp6 group using the HPAI H5N8 virus (A/chicken/Egypt/v1526/2020). Each challenged chicken was inoculated intranasally (IN) with 100 µL containing 106 egg infective dose at 50% (EID50)/chicken, equivalent to 100 chicken lethal dose at 50% (CLD50). All chickens were subjected to daily observation and monitoring for 10 days post challenge (PC) in order to report clinical signs and also to record mortality and detection of virus shedding titer.
Clinical data
Daily observation of all experimental groups was carried out to report any clinical signs or record any mortality throughout the experimental period (41 days).
Evaluation of potency of avian influenza vaccines
The potency of AIV vaccines was evaluated by testing their ability to induce seroconversion in experimentally inoculated chicks.
Ten individual serum samples corresponding to 10 blood samples during the immunization phase (at 1st, 10th, 17th, 24st and 31st DO) were collected from each group (Gps1-5), as well as the control negative group (Gp7). The waning up of the maternally derived antibodies was examined in serum samples from Gp7. Hemagglutination inhibition (HI) tests were conducted using a heterologous inactivated HPAIV A/chicken/Egypt/v1526/2020 (H5N8) antigen to detect H5 clade 2.3.4.4b specific antibodies. These were expected to be induced by post-vaccination responses against inactivated vaccines. The antigen was adjusted to 4 hemagglutinating units according to international standards.12 Arithmetic means of HI titers were expressed as reciprocal log2 and HI at a dilution ≥ 24 was considered for AIV-positive specific antibodies
The seroconversion (seropositivity) rate was estimated as the proportion of chickens with positive HI titers (≤24) and was calculated using the following formula:
where n = number positive with HI titers of ≤24; N = total randomly selected and tested number in the group.
Evaluation of viral load by quantitative real-time reverse transcriptase PCR
The shedding of challenge virus from chickens from vaccinated challenged and non-vaccinated challenged groups was monitored by quantitative real-time reverse transcriptase PCR (qRT-PCR) to assess the effect of vaccination on respiratory shedding of the virus.
The specimens comprised 10 individual oropharyngeal swabs from each challenged group collected onto dry swabs. Sampling was done at 3th, 5th, 7th and 10th days PC. The swabs were eluted by vortexing in 1 mL of PBS + 0.1% of an antibiotics stock solution (penicillin, 100,000 units; streptomycin, 100 mg/mL) and kept frozen at -70 ℃ until use. RNA was extracted and detected by qRT-PCR using a primer set and probe specific for the influenza matrix gene.13 The estimated viral shedding concentration in the specimens was extrapolated from the Cq values using a standard curve which was constructed from 10-fold serial dilutions of the challenge material, similarly to what has been done by others.14 Results were expressed as log10 number of copies/PCR reaction.
The reduction in virus shedding titers from respiratory tract should be at a minimum of 2 log10 (100-fold) less in vaccinated challenged chicken group compared to non-vaccinated challenged group;15 which is considered as a minimum requirement for vaccine efficacy.
Mean shedding titer = sum of shedding titer/number of shedders birds (10 from each group).
Data management and analysis
The collected data were revised, coded, tabulated and introduced to a personal computer using Statistical package for Social Science (SPSS 27). Data were presented and suitable analysis was done according to the type of data obtained for each parameter. The descriptive statistics included mean, standard deviation (± SD) and range for parametric numerical data, while median and interquartile range (IQR) for non-parametric numerical data. Analytical statistics included ANOVA test (used to assess the statistical significance of the difference between more than two study group means), the Kruskal-Wallis test (used to assess the statistical significance of the difference between more than two study group ordinal variables), repeated measure ANOVA test (assess the statistical significance of the difference between means measured more than two times for the same study group) and Post Hoc Test (used for comparisons of all possible pairs of group means). P- value means level of significance: P>0.05, non-significant (NS) and P< 0.05, significant (S).
Results
Genetic relatedness between the challenge virus and vaccine seed strains
Nucleotide sequence identity percentage of the HA genes between the challenge virus and the vaccine seeds revealed that Re-14 strain and EGY2018/H5N8 strain showed the highest identity percentage with 97.83% and 96.84%, respectively; while, the other vaccines strains (Re-5 strain, Re-8 strain, Re-6 strain, Re-13 strain, EGY2016/H5N1 strain, EGY2017/H5N1 strain, and Potsdam/H5N2) showed identity percentages 92.13%, 91.01%, 90.51%, 90.49%, 87.87%, 87.65%, and 86.60% with the challenge virus, respectively, as shown in Table 1.
Table 1 Nucleotide sequence identity percentage of the HA antigens of the challenge virus compared to some commercially available H5 vaccines used in Egypt.
| Group No. | Vaccine tradename | Vaccine seed virus | Subtype | Abbreviation | Clade/Lineage | Identity percentage compared to challenge virus H5N8 |
|---|---|---|---|---|---|---|
| Gp1 | Re-13 & Re-14 vaccine | A/duck/Fujian/S1424/2020 | H5N6 | Re-13 | clade 2.3.4.4h | 90.49% |
| A/whooper swan/Shanxi/4-1/2020 | H5N8 | Re-14 | clade 2.3.4.4b | 97.83% | ||
| Gp2 | Re-5 vaccine | A/duck/Anhui/1/2006 | H5N1 | Re-5 | clade 2.3.4 | 92.13% |
| Gp3 | Re-6 & Re-8 vaccine | A/duck/Guangdong/s1322/10 | H5N1 | Re-6 | Clade 2.3.2.1 | 90.51% |
| A/Chicken/Guizhou/4/13 | H5N1 | Re-8 | clade 2.3.4.4g | 91.01% | ||
| Gp4 | Nobilis vaccine | A/duck/Potsdam/1402-6/1986 | H5N2 | Potsdam/H5N2 | Eurasian | 86.60% |
| Gp5 | MEFLUVAC vaccine | RGA/chicken/ME-2018 | H5N8 | EGY18/H5N8 | clade 2.3.4.4b | 96.84% |
| RGA/CHICKEN/Egypt/ME1010/2016 | H5N1 | EGY16/H5N1 | clade 2.2.1.1 | 87.87% | ||
| A/Chicken/Egypt/ RG-173CAL/2017 | H5N1 | EGY17/H5N1 | clade 2.2.1.2 | 87.65% |
Clinical signs
Pre-challenge period
Chickens in all groups in their first 31 days of life (which corresponds to the pre-challenge or immunization period) were characterized as healthy by clinical inspection.
Challenge period (from day 31 to day 41)
In the non-vaccinated challenged group (Gp6), all chickens developed clinical illness following the challenge, exhibiting symptoms of HPAI including hemorrhage on the legs and shanks, depression, ruffled feathers, cyanosis of the combs and beaks, nervous signs (tremors, convulsions, incoordination), respiratory signs (nasal discharges and breathing difficulties) and diarrhea beginning 24 hours after challenge. Mortality begun on the third day of challenge and by the fourth day all chickens were found dead.
While in the vaccinated groups (Gp1 to Gp5), the majority of chickens remained healthy, with the exception of a small number of chickens that developed clinical illness, and began exhibiting slight depression, ruffled feathers and mild respiratory signs (nasal discharges and rales), several days later than the non-vaccinated challenged chickens. Following that, these sicker chickens developed severe respiratory signs, cyanosis of beak and comb and severe nervous signs, and finally were found dead. Mortality begun the fifth day of the challenge and by the ninth day, there were no more dead or diseased chickens.
Seroconversion
Monitoring of maternally-derived antibodies
During the first day, each tested chicken in the five vaccinated groups and the control group were 100% seropositive.
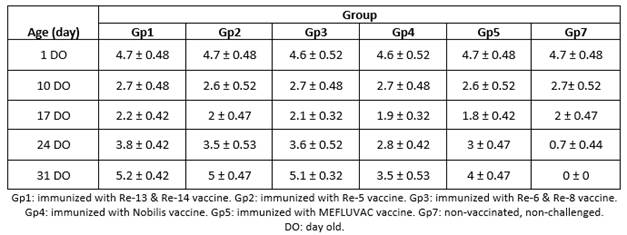
At one DO, the mean HI titers of the maternally-derived antibodies (MDAs) ranged from 4.6 to 4.7 log in all groups, using a heterologous inactivated HPAIV H5N8 antigen. Then, the MDA titers decreased gradually until the second week in the five vaccinated groups. But in the control group, the MDA titers waned up nearly at the fourth week of age, as shown in Table 2.
Humoral response to vaccination
At 24 and 31 DO, sera collected from all vaccinated groups (from Gp1 to Gp5) and tested against heterologous inactivated HPAI H5N8 challenge antigen revealed detectable HI antibody response to vaccination at those ages. At time of challenge (31 DO), the vaccinated group exhibiting the highest mean level of HI titer was Gp1 (with a titer of 5.2 log2), followed by Gp3 (5.1 log2), then Gp2 (5 log2), following that Gp5 (4 log2) and, finally, Gp4 exhibited the lowest HI titer (3.5 log2), as shown in Table 2.
Protective efficacy following HPAI H5N8 challenge
The efficacy of the vaccines was evaluated based on protection against mortality after challenge and reduction of viral shedding. All non-vaccinated challenged chickens showed no protection against the challenge (protection level 0%); their mortality started on day 3 and ended on day 4 PC. On the other hand, in the vaccinated challenged groups, the highest protection level was observed in chickens that received the Re-13 & Re-14 vaccine (95%), followed by those that received Re-5 (90%) and Re-6 & RE-8 (90%) vaccines, then the MEFLUVAC vaccine recipients showed 80% protection and, finally, chickens that received Nobilis vaccine showed the lowest level of protection (70%), as shown in Table 3 and Figure 1.
AIV shedding in oropharyngeal swabs
Among all the challenged groups, the highest viral shedding titer was obtained for the positive control group (Gp6) at 3 days PC (5.4 log10). At the same time, compared to the positive control group, the five vaccinated challenged groups shed significantly less challenge virus with a reduction of 3.8, 3.55, 3.7, 2.85 and 3.3 log10, respectively. Moreover, there was a statistically significant difference between vaccinated groups. Chickens in Gp1, Gp2, Gp3 and Gp5 displayed significant reductions in H5N8 viral shedding titers compared to Gp4 on each day PC. In addition, chickens in Gp1 and Gp3 comparing to that of Gp5 displayed significant reductions in viral shedding. In contrast, no significant difference in shedding levels were found between Gp1, Gp2 and Gp3. At 3rd day, PC results revealed a higher rate of virus shed for Gp4 (3.9 log10) and lower titers were recorded for Gp1 (2.8 log10); at 5th day PC, there was a higher virus shedding titer for Gp4 (3 log10) and lower shedding titers were detected for Gp1 (1.8 log10); at day 7 PC, results were significantly different with a high titer for Gp4 (1.8 log10) and the low titer was presented in Gp1 (1.1 log10), as shown in Table 3.
Table 3 Mean viral load of challenge virus plus standard deviation (log10 EID50/mL±SD) in vaccinated and non-vaccinated groups on days 3, 5, 7 and 10 PC with the HPAI H5N8 virus clade 2.3.4.4b (A/chicken/Egypt/ v1526/2020) and their protection percentages.
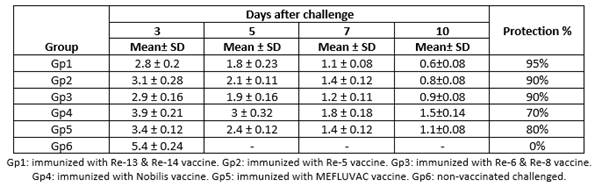
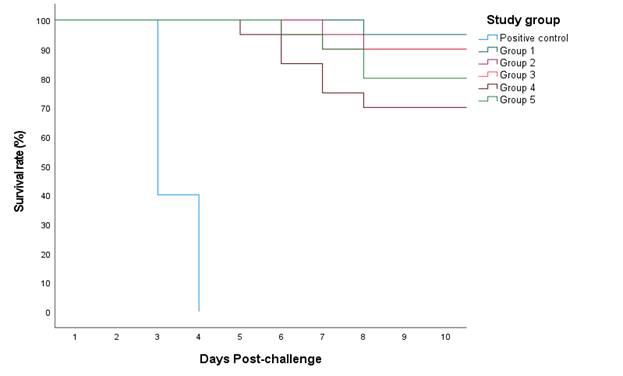
Fig. 1 Time course of mortality in vaccinated and non-vaccinated groups after challenge with the clade 2.3.4.4b HPAI virus strain A/chicken/Egypt/ v1526/2020 (H5N8). Vaccinated groups included Group 1 immunized with Re-13 & Re-14 vaccine, Group 2 immunized with Re-5 vaccine, Group 3 immunized with Re-6 & Re-8 vaccine, Group 4 immunized with Nobilis vaccine and Group 5 immunized with MEFLUVAC vaccine. Positive control: non-vaccinated.
Discussion
The main approaches of controlling HPAI in poultry usually include rapid eradication, animal movement restriction rules and early warning passive surveillance technologies. These strategies might not be enough to prevent introduction of the virus (mainly from infected wild birds) and dissemination within the commercial poultry sector, as recent and recurrent epizootics caused by different A/goose/Guang dong/1/1996-lineage HPAI A(H5Nx) viruses still happened. So, it is necessary to regularly develop and test vaccines that offer effective protection against HPAI (preventing mortality and virus shedding); this should be done regularly to match the emerging circulating virus strains in order to implement complementary preventive vaccination against HPAI in poultry.16
In the present study, the protective efficacy of five different types of commercially available inactivated avian influenza vaccines was assessed in broiler chickens carrying MDAs against HPAI H5N8 virus belonging to major HA clade 2.3.4.4b that circulates in Egypt, and is known to have previously caused severe clinical signs and 100% mortality in non-vaccinated chickens.
According to the results, the Gp1 group that received the Re-13 & Re-14 vaccine showed the highest protection level (95%), the highest HI titer (5.2 log2) and a significant reduction in viral shedding titer and this may be explained by considering that the vaccine is made up of two seed strains: the Re-14 strain, which is H5N8 subtype clade 2.3.4.4b and closely related to the challenge H5N8 clade 2.3.4.4b with identity percentage (97.83%) and the Re-13 strain, which is H5N6 subtype clade 2.3.4.4h, which raises the antigenic mass of H5. These results were in agreement with those of other authors17,18 who reported that the vaccine produced with the seed viruses H5-Re13 and H5-Re14 carrying the HA and NA genes of a newly detected H5N6 virus and H5N8 virus, respectively, provided complete protection against challenge with H5N8 virus bearing the clade 2.3.4.4b in chickens.
Subsequently, we found that Gp2 and Gp3 groups immunized with Re-5 and Re-6 & Re-8 vaccines, respectively, attained 90% protection levels for both of the groups, along with demonstrating significantly high HI titers (5 and 5.1 log2, respectively) and significantly reduced viral shedding titers. These could be related to the fact that the Re-5 vaccine is made up of a seed strain (Re-5 strain, H5N1 subtype clade 2.3.4) closely related to the challenge H5N8 clade 2.3.4.4b with identity percentage of 92.13% and the Re-6 & Re-8 vaccine is made up of two seed strains (the Re-6 and Re-8 strains) which are H5N1 subtype bearing clade 2.3.2.1 and clade 2.3.4.4g, respectively and these strains were found to be closely related to the challenge H5N8 clade 2.3.4.4b with identity percentage 90.51% and 91.01%, respectively and also believed to increase the H5 antigenic mass. These findings were supported by other studies18,19 and disagreed with the findings of Kilany, et al.20
Furthermore, the Gp5 group which received MEFLUVAC vaccine achieved a protection level of 80%, a significant high HI titer (4 log2) and a significant reduction in viral shedding titer; these findings are possibly explained by the fact that the vaccine consists of three seed strains: one of them is EGY18/H5N8 clade 2.3.4.4b, which is closely related to the challenge virus and demonstrated 96.84% identity percentage and the other two strains are Egy2016/H5N1 clade 2.2.1.1 and Egy2017/H5N1 clade 2.2.1.2, which expected to increase the antigenic mass of H5. These results are consistent with those obtained in another study.10
Finally, the Gp4 group, which demonstrated the least protection level of 70%, received the Nobilis vaccine; it also had low levels of HI titers (3.5 log2) and reduction of viral shedding titers. This could be attributed to the genetic differences between the vaccinal strain and the challenge virus, as it demonstrated 86.6% similarity in genetic relatedness between the vaccinal strain and the challenge virus; these results were in agreement with findings of other authors.19
Conclusion
The present study, aimed to assess the efficacy of five commercially inactivated H5 vaccines belonging to a different lineage against challenge with HPAI H5N8 virus in broiler chickens in Egypt, revealed that the Re-13 & Re-14 vaccine gives a superior humoral immune response and lower virus shedding resulting in a higher protection level, whereas the Nobilis vaccine achieves the lowest humoral immune response and protection percentage. This could show the importance of continuously evaluating and updating avian influenza poultry vaccines in Egypt, as vaccination is used as an effective method to prevent and control of AIV spread.