Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.1 La Habana ene.-mar. 2009
REPORT
Mutated variant of human vascular endothelial growth factor as a vaccine candidate for cancer immunotherapy*
Mónica Bequet-Romero, Yanelys Morera, Marta Ayala, Boris E Acevedo, Humberto Lamdan, Jorge V Gavilondo
Recombinant Antibodies Laboratory, Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 and 190, Playa, POBox 6162, Havana 10600, Cuba
INTRODUCTION
The finding that neo-angiogenesis, –the production of new blood vessels from pre-existing ones–, is an essential mechanism for tumor development and progression (1) has produced a totally new modality for cancer treatment: anti-angiogenic therapy (1). Novel anti-angiogenic cancer therapies based on synthetic and natural molecules target pro-angiogenic growth factors produced by tumors and/or their cell surface receptors in endothelial cells, or even in some cancer cells. Among these growth factors, vascular endothelial growth factor (VEGF) and its receptors have received special attention, due to: a) their central role in endothelial cell physiology and neo-angiogenesis; b) the detection of VEGF at high concentrations in most of the human tumors and their metastasis; (c) its frequent association with a bad prognosis in cancer; and d) the differential nature of tumor angiogenesis, when compared to normal tissues (2).
Anti-angiogenesis as cancer therapy was validated in 2004, when the FDA first approved Bevacizumab (Avastin®, Genentech), a humanized monoclonal antibody that blocks the interaction of circulating VEGFA and VEGF receptors, for the treatment of metastatic colorectal cancer (3).In the following years Bevacizumab has been also approved for the treatment of non-small cell lung carcinoma (4) and metastatic breast cancer (5), and is under phase I, II, and III clinical trials in more than thirty other tumor types. Several other antibodies that block VEGF-VEGFR2 interaction are also under intensive preclinical and clinical investigations (6).
An exciting new approach for antiangiogenic cancer therapy involves active specific immunotherapy. Antiangiogenic cancer vaccines could hopefully not only elicit endogenous antibodies that neutralize VEGF and its receptors, but also induce cytotoxic T lymphocytes- mediated tumor destroying mechanisms. Collateral effects of such vaccines could also be milder than those elicited by externally infused anti-VEGF antibodies.
Experimental vaccination using xenogeneic (7) and autologous (8, 9) endothelial cells have initially validated the likelihood of this strategy. But, the heterogeneity of these vaccine preparations have encouraged studies with more defined vaccine antigens, namely, pro-angiogenic factors and their receptors. Xenogeneic (10) and autologous VEGF (11), VEGF receptor 2 (12), in the form of protein or naked DNA vaccines, or infused as antigen-loaded dendritic cells, have resulted in antitumoral and antiangiogenic effects in cancer animal models.
Wei et al. (10) using xenogeneic VEGF165 DNA as antigen, and Rad et al. (13), immunizing with autologous VEGF164 protein kinoids, have reported the induction of an antibody-mediated VEGF neutralizing response that led to anti-tumor effects, but with no evidences of breaking T-cell tolerance to this growth factor. On the contrary, Kamstock et al., immunizing an outbreed dog population affected by advanced sarcomas, demonstrated a 30% tumor response rate using human VEGF165 protein mixed with a previously prepared liposome-DNA complex, but found no relevant antibody titers to canine VEGF (11).
Within this rising field of investigation, our group was very early involved in the development of an improved VEGF vaccine strategy, having as centerpiece the use of a functional mutant of the autologous VEGF molecule (or a very high homologous one), impaired for binding to the VEGFR2, as antigen.
Our preliminary results demonstrated the feasibility of using a human VEGF variant impaired for VEGFR2 binding as a vaccine antigen in a naked DNA format as an antitumoral approach in mice and provide an insight on the immune mechanism involved (14). Further work was directed to the development of a new candidate vaccine using a recombinant antigen produced as a fusion protein containing the mutated human VEGF and an amino terminal sequence of Neisseria Meningitidis P64K protein. The antigen was tested with adjuvants of different chemical nature clinically proven or under advanced preclinical testing. With this vaccine candidate, we showed the induction of anti-tumor effects in the C57Bl/6 melanoma B16-F10 tumor system, with the production in mice of neutralizing antibodies against both human and mouse VEGF, and specific T cell responses against murine tumor cells that produce VEGF (15).
ANTI-TUMOR EFFECT OF A HUMAN VEGF GENE VARIANT IMPAIRED FOR VEGFR2 BINDING ADMINISTERED TO SYNGENIC MICE AS A NAKED DNA VACCINE
The human VEGF121 gene with its native signal peptide was PCR-amplified and cloned into pMAE5δ5, a vector designed for naked DNA immunization and bearing 5 CpG motifs (14). The VEGFR2 binding zone of the VEGF gene was either preserved or disrupted, the latter one by overlapping PCR substitution of the bases corresponding to the aminoacids R82, K84 and H86, by those coding for glutamic acid (E). Resulting constructions were named pM-VEGF and pM-VEGFKDR-, respectively. DNA for vaccination was purified using Giga Endo-free columns (QIAGEN) and formulated in phosphate buffered saline (PBS) to a final concentration of 2 mg/mL and stored at -20 °C in aliquots.
DNA immunization was conducted by injecting 50 µL of test plasmid DNA in PBS by the left quadriceps intramuscular (i.m.) route and administered every day for 4 weeks. Subcutaneous (s.c.) tumor challenge was done a week after the last immunization, as well as the evaluations of the tumoral and cellular immune responses.
Prophylactic immunization with pM-VEGF significantly delayed tumor development for melanoma B16-F10 (p = 0.0201), reduced tumor growth kinetics (p < 0.01), and increased C57Bl/6 mouse survival (p = 0.0229), as compared to the control group receiving the empty vector. A similar effect was observed in the same mouse strain using the lung carcinoma model TC-1, where from day 28, after tumor challenge, a significant reduction on tumor volume was detected, as well as, a significant increase on survival (p = 0.0428) of the pM-VEGF treated animals (14). These results demonstrated that it was possible to induce an antitumoral response by administering naked DNA encoding for a VEGF molecule highly homologous to its murine counterpart (89%).
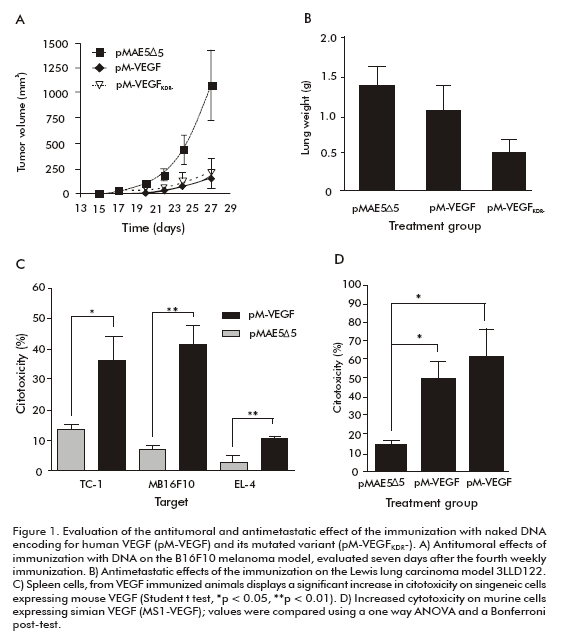
To avoid concerns of regulatory agencies for the clinical testing of a VEGF-based vaccine, and taking into consideration that the pro-angiogenic potential of VEGF is directly related to its binding to VEGFR2 (2), we mutated the human VEGF121 gene (hVEGFKDR-) encoding of key residues for receptor recognition (16). The mutations introduced in the human VEGF121 gene affected the so-called loop of the 80s of VEGF. This region has been reported to be recognized or sterically blocked by several neutralizing antibodies (17), and such mutation in an antigen could hamper the production of this type of immunoglobulins. Nevertheless, we hypothesized that other biologically relevant epitopes in VEGF would expose. Naked DNA immunization of mice with the pM-VEGFKDR- mutated gene molecule evidenced that the anti-tumor effect was still present when animals were challenged with melanoma B16-F10 (Figure 1A). Furthermore, the comparative evaluation of the anti-metastatic effect of the immunization with pM-VEGF and pM-VEGFKDR showed that both genes led to a significant reduction in the number of lung metastases, using the spontaneous metastases Lewis lung carcinoma model, where footpad tumors were removed after sustained growth and lungs were later on examined for metastatic dissemination (Figure 1B) (14).
No anti-human VEGF121 antibody titers were detected by a specific ELISA after prophylactic immunization using either pM-VEGF or pM-VEGFKDR-, but freshly isolated spleen cells from vaccinated mice induced significant direct in vitro lysis of three murine VEGF-producer tumor cell lines, without prior IL-2 stimulation (Figure 1C), as evaluated using a LDH release assay. In addition, spleen cells from such animals showed a significant increase in IFN-γ secretion after co-culturing for 24 hours with EL-4 target tumor cells, comparing spleen cells obtained from empty pMAE5∆5 vector-treated mice. Moreover, cytolysis of mouse endothelial cells (MS-1- VEGF) transfected with monkey VEGF isoform 121 was seen after spleen cells of pM-VEGF or pM-VEGFKDR- immunized animals were stimulated by low amounts of IL-2 and antigen (Figure 1D). In the absence of a humoral response to VEGF, these result demonstrated for the first time the possibility of activating the cellular arm of the immune response in C57Bl/6 mice to achieve an antitumoral effect.
DEVELOPMENT AND EXPERIMENTAL TESTING OF A VACCINE CANDIDATE BASED ON A RECOMBINANT MUTATED HUMAN VEGF AND A CLINICALLY TESTED ADJUVANT
Taking into account the reported low efficiency of naked DNA vaccination in humans, and the need of enhancing the anti-tumor response to immunization via the development of both neutralizing antibodies and specific cytotoxic T lymphocytes, our work moved towards the production of a suitable recombinant antigen in Escherichia coli. The first expression system used was the pGEX-6P plasmid GST fusion system. The human VEGF121, and the corresponding mouse VEGF isoform 120, were cloned, expressed, and purified using glutathione-Sepharose affinity chromatography. Both GST fusion protein preparations were able to elicit high antibody titres in mice after immunization, similar to commercially available human VEGF, using Freunds adjuvant (18).
To produce a vaccine antigen feasible for clinical trials, the mutated human VEGF isoform 121 gene was cloned. The recombinant antigen P64k- VEGFKDR-, was designed as a fusion protein where the mutated human VEGF is genetically coupled at its N-terminus to the first 47 aminoacids of the N. meningitidis P64K protein. Protein yileds of 60 mg/L were obtained from non-optimized bacterial cultures, a promising figure in terms of scaling up (15).
Experimental anti-tumor activity of this recombinant antigen was tested with three adjuvants of different chemical composition, already employed in humans, or having strong preclinical data available: aluminum hydroxide, very small sized proteoliposomes (VSSP; Center of Molecular Immunology, CIM, Havana) and dimethyldioctadecil ammonium/ trehalose 6,6 dibehenate (CAF01; Serum Institute, Denmark). The vaccination scheme involved weekly s.c. injections of 100 µg of antigen and adjuvant, in the form of three prophylactic and three therapeutic immunizations. The combination of P64khVEGFKDR- and the three adjuvants demonstrated positive anti-tumor effects against C57Bl/6 melanoma B16-F10 in terms of extended time for the appearance of palpable tumors. Effects of vaccination on tumor growth kinetics were evident when performed the P64k-hVEGFKDR- + VSSP combination, only partial when the adjuvant was changed to Alum, and not detected with the P64k-hVEGFKDR- + CAF01. However, a significant increase in animal survival was observed for all the experimental settings testing antigen plus adjuvant, when compared with the control animals (Figure 2A-C) (15).
Immunization with the P64k-hVEGFKDR- protein antigen and all the adjuvants tested resulted in IgG antibodies that recognized wild type human and murine VEGF in a correlated manner (Figure 3A). In vitro experiments were performed to test whether the antibodies raised by vaccination were capable of neutralizing the stimulatory effect of human VEGF on the proliferation of human umbilical cord endothelial cells (HuVEC). Taking as example antigen plus alum, or antigen plus VSSP (that resulted in a better anti-tumor response), we found that pooled sera from these two treatment groups blocked the proliferation of HuVEC, and sera from animals immunized with P64k-hVEGFKDR- plus VSSP were better at blocking (Figure 3B). Moreover, when the group of animals immunized with antigen plus VSSP was stratified according to the effect of vaccination in tumor growth at a given point of time, sera coming from mice showing lower tumor volumes representative of a higher effect, consistently produced a stronger inhibition of the stimulatory effect of human VEGF on HuVEC (Figure 3C). An ELISA assay was developed to test the ability of sera to block VEGF-VEGFR2 (KDR) interaction. The ability of the antibodies raised by vaccination to interfere the binding of soluble KDRFc to VEGF adsorbed to the solid phase was confirmed by this alternative method (15).
To explore the involvement of direct cell cytotoxicity in the overall antitumor response seen in our experiments, C57Bl/6 mice immunized with antigen plus VSSP were depleted of CD8+ T cells with specific monoclonal antibodies during and after the immunization schedule. We found that CD8+ T cells removal significantly abrogated the anti-tumor effect, an indication of a potential involvement of a cellular response in the antitumoral effect of our VEGF targeted immunotherapy. Also, spleen cells of immunized mice were able to provoke lyses of CFSE-charged tumor cells, as demonstrated by FACS (15).
CONCLUSIONS
Our results demonstrated that it is possible to produce IgG antibodies that neutralize the pro-angiogenic effects of human and murine VEGF, induce specific cytotoxic T cell responses, and elicit consequent antitumor effects in mice via vaccination with a mutated human VEGF molecule. In our way to clinical trials, we have developed and produced the antigen as a recombinant protein in E. coli, and tested with success using clinically relevant adjuvants in different experimental settings. Our novel active immunotherapy approach to cancer combines anti-angiogenesis, by selective antibody removal/blocking of soluble proangiogenic VEGF produced by tumors, and the development of an anti-tumor T cell response.
ACKNOWLEDGEMENTS
We thank to the following collaborators of the CIGB for their contribution to this work: Yordanka Soria, Dioslaida Urquiza, Aracelys Blanco, Omar López, Ernesto Galbán, Ariel Vázquez, Myladis Limonta, Victoria M. Lugo, Daniel Yero, Isis del Carmen Torrens, Leonardo Canaan, Hansel Bell, Ernesto M González, Jorge Sánchez and Maelys Miyares; and to Dr. Daniel Yero from the Finlay Institute.
REFERENCES
1. Folkman J. Angiogenesis: an organizing principle for drug discovery Nat Rev Drug Discov 2007;6:273-286.
2. Ferrara N. VEGF as a therapeutic target in cancer. Oncology 2005;69 Suppl 3:11- 6.
3. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-2342.
4. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-2550.
5. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-2676.
6. Posey JA, Ng TC, Yang B, Khazaeli MB, Carpenter MD, Fox F, et al. A phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinoma. Clin Cancer Res 2003;9:1323- 1332.
7. Wei YQ, Wang QR, Zhao X, Yang L, Tian L, Lu Y, et al. Immunotherapy of tumors with xenogeneic endothelial cells as a vaccine. Nat Med 2000;6:1160-1166.
8. Chen XY, Zhang W, Zhang W, Mirabet V, Carbonell F, Castell JV, et al. Vaccination with viable human umbilical vein endothelial cells prevents metastatic tumors by attack on tumor vasculature with both cellular and humoral immunity. Clin Cancer Res 2006;12:5834-5840.
9. Okaji Y, Tsuno NH, Kitayama J, Saito S, Takahashi T, Kawai K, et al. Vaccination with autologous endothelium inhibits angiogenesis and metastasis of colon cancer through autoimmunity. Cancer Sci 2004;95:85-90.
10. Wei YQ, Huang MJ, Yang L, Zhao X, Tian L, Lu Y, et al. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci USA 2001;98:11545-50.
11. Kamstock D, Elmslie R, Thamm D, Dow S. Evaluation of a xenogeneic VEGF vaccine in dogs with soft tissue sarcoma. Cancer Immunol Immunother 2007;56:1299-309.
12. Li Y, Wang MN, Li H, King KD, Bassi R, Sun H, et al. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med 2002;195:1575-84.
13. Rad FH, Le BH, Paturance S, Larcier P, Genne P, Ryffel B, et al. VEGF kinoid vaccine, a therapeutic approach against tumor angiogenesis and metastases. Proc Natl Acad Sci USA 2007;104:2837-42.
14. Bequet-Romero M, Ayala M, Acevedo BE, Rodríguez EG, Ocejo OL, Torrens I, et al. Prophylactic naked DNA vaccination with the human vascular endothelial growth factor induces an anti-tumor response in C57Bl/6 mice. Angiogenesis 2007;10:23-34.
15. Morera Y, Bequet-Romero M, Ayala M, Lamdán H, Agger EM, Andersen P, et al. Antitumoral effect of active immunotherapy in C57BL/6 mice using a recombinant human VEGF protein as antigen and three chemically unrelated adjuvants. Angiogenesis 2008;11:381-93.
16. Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, et al. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem 1996;271:5638-46.
17. Kim KJ, Li B, Houck K, Winer J, Ferrara N. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors 1992;7:53-64.
18. Morera Y, Lamdan H, Bequet M, Ayala, G, et al. Rojas, Y. Muñoz, et al. Biologically active vascular endothelial growth factor as a bacterial recombinant glutathione S transferase fusion protein. Biotechnol Appl Biochem 2006;44:45-53.
*This work received the Award of the National Academy of Sciences of Cuba for the year 2008.
Mónica Bequet-Romero. Recombinant Antibodies Laboratory, Center for Genetic Engineering and Biotechnology, CIGB. Ave. 31 / 158 and 190, Playa, POBox 6162, Havana 10600, Havana, Cuba. E-mail: monica.bequet@cigb.edu.cu