Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.26 n.1 La Habana ene.-mar. 2009
REPORT
New evidence of the development of dengue hemorrhagic fever during the dengue 3 epidemic in Cuba *
Mayling Álvarez, MariaG Guzmán, Alequis Pavón-Oro, Lídice Bernardo, Rosmari Rodríguez, Luis Morier, Susana Vázquez, Gustavo Kourí
Arbovirus Laboratory, Department of Virology, PAHO/WHO Collaborating Center for Viral Diseases, Tropical Medicine Institute. Autopista Novia del Mediodía Km 6 ½, La Lisa, PO Box 601, Marianao 13, Havana, Cuba
ABSTRACT
For the first time this paper defines some of the factors involved in the development of dengue hemorrhagic fever during the dengue-3 epidemic in Cuba. The secondary infection is confirmed to be the most important risk factor in that epidemic, after 24 years of the primary infection with dengue-1, in contrast to the infection sequence dengue- 2/dengue-3 that showed no relationship. Tertiary infection was also related to the development of dengue hemorrhagic fever. The neutralizing antibody titers were different for strains of dengue-3 of the same genotype in a panel of sera of late convalescent patients with both clinical epidemic conditions. Viremia was longer in the primary than in secondary infection; the first neutralizing antibodies were not detected during acute phase, while in the secondary infection the neutralizing antibodies were detected after the fifth day, coinciding with the decline of the viremia. In general, neutralizing antibodies reached lower levels in dengue fever patients, compared to those who developed dengue hemorrhagic fever.
Keywords: Cuba, dengue 3 epidemic, dengue hemorrhagic fever, secondary infection, neutralizing antibodies, viremia, viral infection sequence, genotypes.
INTRODUCTION
After more than 50 years without any outbreaks, dengue has gradually increased in most of North and South American countries, with more than one circulating serotype and frequent epidemics. In this context, the epidemic in Cuba was considered to be exceptional in the period of 1970-2002, due to the timing of the four epidemics and the outbreak that occurred. These were eradicated in a short period of time, also preventing the disease to become endemic. The four epidemics were: 1) dengue 1 virus (DEN-1) American genotype epidemic of 1977-1978, which extended throughout the entire island and only caused dengue fever (DF) disease (1); 2) DEN-2 epidemic of 1981, affecting the entire country with dengue hemorrhagic fever (DHF); 3) DEN-2 epidemic of 1997, similar to that of 1981 but confined to the city of Santiago de Cuba; and 4) DEN-3 epidemic of 2001-2002 in Havana city, with the presence, for the first time, of the DEN-3 virus in Cuba. Both DEN-2 epidemics occurred 4 and 20 years, after the initial DEN-1 epidemic, respectively (2, 3), and the last three epidemics showed an increased proportion of DHF/DF cases and DHF-related deaths during their progression (4, 5).
Additionally, there was a small short lasting outbreak in Havana city in the year 2000, and only 138 DF cases were detected, caused by DEN-3 and DEN- 4 strains (5).
Our country has been free of endemic dengue disease since 2002, a significantly different scenario from that of the rest of the countries of the area.
Dengue virus pathogenesis has always been controversial since the initial studies that pointed to the secondary infection as the main risk factor, among others related to virulence (6, 7). It was not until 1987, after the Cuban epidemic of 1981, that the group of Dr. Gustavo Kourí outlined a comprehensive hypothesis on the development of DHF (8). It includes the interactions of three groups of factors: the host, the virus and epidemiological aspects. Among the main individual factors are: sex, race, chronic illness and, basically the pre-existing antibodies.
Defining these factors is therefore crucial for managing the severe illness during a DEN-3 epidemic and because of the probability of several sequences of infection. We then assessed the role of a prior immunity against DEN-1 and DEN-2 serotypes, evaluating the behavior of neutralizing antibodies against different strains of DEN-3, and determining its kinetics in a group of patients with an accurately known prior immunity; this was done in relation to the type of infection, the clinical evolution and the development of the viremia.
RESULTS
Characterization of the secondary and tertiary infection in the DEN-3 Cuban epidemic of 2001-2002
In the DEN-3 Cuban epidemic, 78 cases of DHF were reported. Of these, 75 were hospitalized in our institution (IPK), and their serological response was studied in samples collected during hospitalization. Also, in a small group of cases of both clinical outcomes (DF and DHF), we tried to reconstruct the history of the infection by analyzing the neutralizing antibody responses in the post-convalescent sera.
First, we analyzed the samples of 57 patients (73.2%) diagnosed as DHF, of which all were shown to be infected with more than one dengue serotype. This suggested that the severity of the illness was associated to the infection of more than one virus serotype, under the new epidemic conditions. In parallel, the neutralizing antibody responses were also studied in sera collected from 17 DHF/dengue shock syndrome (DSS) patients in the acute and late convalescent phases of infection. A DEN-1/DEN-3 infection sequence was observed in eleven of them, while the remaining 6 suffered from a tertiary infection. Neutralizing antibody titers were also studied in sera from 37 additional DHF/DSS cases collected between months 18 to 24 after developing the illness. Three cases (8.1%) showed a pattern consistent with a DEN-3 primary infection, 30 (81.1%) showed neutralizing antibody titers against DEN-1 and DEN- 3, suggesting a secondary infection, and 4 (10.8%) showed high antibody titers against DEN-1, DEN-2 and DEN-3 viruses, indicating tertiary infection. None of the sera suggested a DEN-2/DEN-3 sequence of infection.
On the other hand, the study of neutralizing antibody titers in 40 sera collected in the late convalescent phase of DEN-3 DF cases in this epidemic clearly indicated a previous infection with DEN-1 in 45% of the cases, with DEN-2 in 15%, and DEN-1 or DEN-2 followed by DEN-3 infections in the remaining 15%. In short, 6 cases were primary (15%) and 34 cases (85%) were secondary DEN-3 infections, respectively. In these 34 secondary infections, 22 (55%) presented the viral infection sequence DEN-1/DEN-3, while 6 (15%) were a tertiary infection (DEN-1/DEN-2/DEN-3; 15%) and 6 (15%) showed the DEN-2/DEN-3 viral infection sequence. All these suggested that a sequential DEN- 2/DEN-3 infection was present in the DF cases.
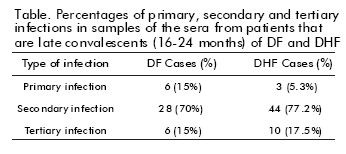
Epidemiological studies have shown that residents in Havana City would be immune to 3 of the 4 dengue serotypes, before the circulation of DEN-3 during the 2001/2002 epidemic. The DEN-1 virus circulated in Cuba during the period 1977-79, while the virus DEN- 2 circulated in 1981 and at some time in or before 1945. It was then assumed that during the 2001/2002 epidemic, a large number of individuals would develop a tertiary infection. Therefore, the adult population of Havana City, previously infected during the epidemics mentioned above, was at risk of suffering from a tertiary infection and developing an asymptomatic infection. In this study, a tertiary infection (DEN-1/ DEN-2/DEN-3) was demonstrated in 10 (17.5%) individuals with health records of severe illness (DHF) during the epidemic and in 6 (15%) with a benign form of the illness (DF). The table summarizes the infection sequences found in the samples coming from both clinical settings.
The study of 73% of the cases of DHF/DSS reported in Havana City in 2001-2002 led to the conclusion that: a) Most of the individuals with DHF (94.7%) had developed an initial primary infection of DEN-1; b) The DEN-1/DEN-3 infection sequence occurred at a mean age of 24 year-old in the DHF/DSS clinical setting; c) Although the sequence of DEN-2/ DEN-3 was able to induce the DF setting, it was not associated to the development of DHF. Most of the DHF/DSS cases were found in people who were infected with DEN-3 virus and had records of DEN-1/ DEN-2 secondary infection from the 1977-79 and 1981 epidemics or the 1945 outbreak. Thus, the development of DEN-3 DHF/DSS cases decades after the primary infection with DEN-1 contributes to the knowledge on its relationship to this entity. It also increases the challenge for the development of dengue vaccines. These results also suggest that primary infection with the DEN-2 virus does not sensitize patients in developing the severe form of the illness (DHF).
Kinetics of the neutralizing antibody response against different DEN-3 strains
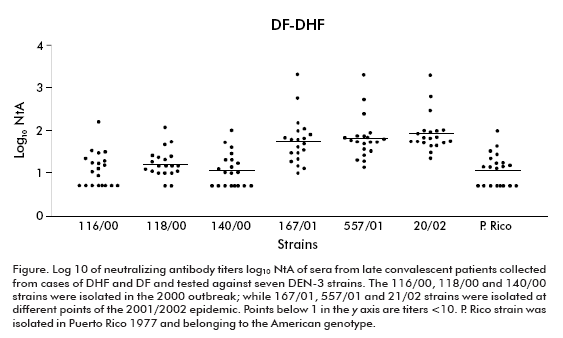
Keeping in mind the epidemic situation of our country and particularly the introduction of the DEN-3 virus in the years 2000 and 2001/2002, we wonder if there could be differences in the neutralizing capacity of sera against DEN-3 strains of different genotypes. The neutralizing capacity was studied in sera of late convalescent individuals immune to DEN-3 during the 2001/2002 epidemic. In our study, three strains isolated from both Cuban outbreaks were included, all belonging to the Asian genotype. A strain of the American genotype isolated in Puerto Rico in 1977 was also included.
The highest neutralizing antibody titers were observed against homologous DEN-3 strains isolated during the 2001/2002 epidemic (p < 0.0001), in DF (p < 0.001) and DHF (p < 0.05) cases.
The lowest titers and the lowest geometric mean titers (GMT) were observed against the DEN-3 strain isolated in the 2000 outbreak and against the Puerto Rican strain, their GMT ranging from 9 (standard deviation ± 2.99) to 18 (standard deviation ± 1.98).
The figure shows the individual results and the standard deviations of the log10 of sera neutralizing antibody titers against all strains.
Considering that protein E is the most relevant antigen for the neutralizing antibody response, changes in its amino acid sequence among the DEN-3 strains could be responsible for these observations. The Cuban strains of both outbreaks (2000 and 2001/2002) showed 3 non-synonymous substitutions: Thr19/Pro, Ile226/Thr and Ala329/Val. Moreover, the last strain isolated during the epidemic of 2001/2002 showed a non-conserved amino acid change in Asp22/Val.
Another interesting finding was the highest level of neutralizing antibodies against the homologous virus strain in sera collected from late convalescent DF patients compared to those of DHF patients. Although it was not possible to define the cause of this observation, it is suggested that some DF cases probably developed more effective immune responses and antibodies with higher affinity, and, consequently, had a better control of the infection than the DHF cases. Hence, this may produce an increased persistence of antibodies with greater affinity against the virus in the sera collected from DF patients.
Here we demonstrated the differences in the neutralizing capacity of the sera against different genotypes and also strains within the same genotype. These results are relevant for the pathogenesis of dengue and for vaccine development.
Kinetics of neutralizing antibodies and their relationship with viremia in 22 patients of the Cuban epidemic of 2001-2002
Sera was collected daily from 22 Cuban adult patients starting at their admission in the hospital during the second or third day after the onset of fever and until the day they were discharged (approximately on day eight), with a final extraction carried out 15 to 30 days later outpatient consultations. Nineteen of them developed DF and the other three had DHF during the DEN-3 2001/2002 epidemic.
Of the 91 samples studied, 36 (39.5%) were positive, being identified as DEN-3 virus; this viral agent was isolated from 20 patients (90.9%).
Viral isolation was positive in samples collected between days 2 and 4 of the illness. The DEN-3 virus was isolated in 100% of the samples collected on the second day of the feverish condition and in 90.4% of those of the third day. During the fourth day, positive cases decreased to 54.5% and only one case (4.5%) was positive on the fifth day.
The virus was not isolated from any of the samples collected from 2 out of 22 patients; however, serological results confirmed dengue infection (presence of IgM antibodies against dengue).
In the cases previously classified as the primary infection, neutralizing antibody levels against the viruses studied were not demonstrated in samples collected between days 2 to 8 after the onset of the disease. In these patients, a sample could not be taken after day 8 of the disease. It is plausible that neutralizing antibody titers were at that moment at levels lower than the 1/10 starting dilution threshold of our technique.
Those patients with a primary infection whose neutralizing antibodies were not detected in the samples studied, showed a greater and more persistent viremia than patients with secondary infection. One-hundred percent of the cases with the primary infection developed the benign form of the illness in spite of having a higher mean duration of the viremia.
In patients with a secondary infection the neutralizing antibody response started to rise at the fifth day of the illness, coinciding with the decline of the viremia. In all DF and DHF cases the highest titers were developed against the DEN-1 serotype. This is based on epitopes shared by serotypes, which are immunodominant and reinforce the anamnestic response against the primary infection. However, 16.7% of the patients with a secondary infection developed DHF. This finding supports previous reports and suggests, as outlined by other authors, that the presence of antibodies against a previous infection sensitizes the patient in the development of the severe form of the illness, thus being a risk factor. The failure of a pre-existent protective mechanism in patients with a secondary infection is responsible for the uncontrolled immunopathogenic processes leading to DHF/DSS.
On comparing the levels of neutralizing antibodies in patients with a secondary infection also having DF and DHF we found differences in the response patterns. In general, neutralizing antibody levels were lower in DF than in DHF patients.
Interestingly, neutralizing antibodies against DEN- 4 peaked only at the 1/30 dilution for DHF patients. Particularly, the levels of neutralizing antibodies against DEN-3 were high in DHF patients compared to those of DF. The increase of neutralizing antibodies in the DHF cases could be given by the strong immune activation resulting from the severe infection. There may also be a lower recognition of the virus by the heterotypic neutralizing antibodies during the viremia in DHF patients, and, therefore, a larger amount of free neutralizing antibodies that can be detected later in the reduction neutralization test.
Although the sequential viral infection could not be determined in secondary cases, since late post-convalescent samples were not taken, these results suggest that most of the patients should have had the first infection for DEN-1 in the years 1977-1979, the most probable sequential infection in these cases being DEN-1/ DEN-3. Both patients of under 22 years of age, when the sample were collected, could not have been infected with DEN-1 and suffered from a primary infection. This result was in agreement with the epidemic situation of Havana, where the disease was not present since 1981 (20 years earlier).
RELEVANCE OF THE STUDY
This was the first study to demonstrate that the immunity against DEN-1 sensitizes the individuals to DHF during a secondary infection by DEN-3, in contrast to the sequential DEN-2/DEN-3 infection that it is not associated to DHF. Differences in the neutralizing capacity of the immune serum against the DEN-3 virus in the presence of DEN-3 strains of the same and different genotypes were also shown. This is relevant for the development of a vaccine candidate and for the knowledge on the etiopathogenesis of the disease.
On the other hand, we established the kinetics of the neutralizing antibody response against the four serotypes in Latin American patients with a DEN-3 infection whose previous immunity was accurately known. Tertiary infection, although progressing in most of the studies as an asymptomatic infection, was shown to be a predisposing factor for the development of DHF.
In summary, we confirmed the multifactorial theory of the development of the severe form of dengue stated more than 20 years ago by the group headed by Dr. Gustavo Kourí. This study offers a practical contribution in the prevention of the presence of severe signs of the disease and particularly for the protection of persons suffering from a primary infection (personalized epidemiological surveillance).
CONCLUSIONS
The sequential DEN-1/DEN-3 and DEN-1/DEN-2/ DEN-3 infections were associated to DHF during the epidemic of DEN-3 in 2001/2002, while the DEN-2/ DEN-3 infection showed no association. The development of the DHF is demonstrated in the course of the tertiary DEN-1/DEN-2/DEN-3 infection. Our results supported a previous immunity to DEN-1 as predisposing the person in the development of DHF 24 years after the primary infection. Differences were demonstrated in the neutralizing capacity of sera against strains of different DEN-3 genotypes. Neutralizing antibody titers were higher against the strain isolated at the end of the 2001/2002 epidemic and in the DF cases. There were also differences in the duration of the viremia and the kinetics of the neutralizing antibodies according to the type of infection and the clinical setting of the disease.
ACKNOWLEDGEMENTS
The authors would like to thank Sheila Cabezas, Msc; Irina Prado, MSc; Yinet Castellanos, MSc; Yamira Caballero, Technician; Dr. Lizet Sánchez, Dr. Angel M Alvarez, Dr. Daniel González and Dr. Osvaldo Castro for their collaboration in parts of this study.
REFERENCES
1. Más P. Dengue fever in Cuba in 1977: some laboratory aspects. In: Dengue in the Caribbean. Scientific Publication No. 375. Washington DC: Pan American Health Organization, 1979:40-3.
2. Kourí GP, Guzmán MG, Bravo Jr, Triana C. Dengue haemorrhagic fever/dengue shock syndrome: lessons from the Cuban epidemic, 1981. Bull World Health Organ 1989;67(4):375-80.
3. Kouri G, Guzmán MG, Valdes L, Carbonel I, del Rosario D, Vázquez S, et al. Reemergence of dengue in Cuba: a 1997 epidemic in Santiago de Cuba. Emerg Infect Dis 1998;4(1):89-92.
4. Guzmán MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vázquez S, et al. Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am J Epidemiol 2000;152(9):793-9.
5. Guzmán MG, Kourí G. Dengue: an update. Lancet Infect Dis 2002;2(1):33-42.
6. Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. II. Clinical laboratory responses to heterologous infection. J Infect Dis 1973;128(1):15-22.
7. Rosen L. The pathogenesis of dengue haemorrhagic fever. A critical appraisal of current hypotheses. S Afr Med J 1986;Suppl Suppl:40-2.
8. Kourí GP, Guzmán MG, Bravo JR. Why dengue haemorrhagic fever in Cuba. 2. An integral analysis. Trans R Soc Trop Med Hyg 1987;81(5):821-3.
*This work received the Award of the National Academy of Sciences of Cuba for the year 2008.
Mayling Álvarez. Arbovirus Laboratory, Department of Virology, PAHO/WHO Collaborating Center for Viral Diseases, Tropical Medicine Institute. Autopista Novia del Mediodía Km 6 ½, La Lisa, PO Box 601, Marianao 13, Havana, Cuba. E-mail: mayling@ipk.sld.cu