Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.27 n.2 La Habana abr.-jun. 2010
REVIEW
Treatment and recovery of Wagner 5 diabetic foot with Heberprot-P
Tratamiento y recuperación del pie diabético grado 5 de la clasificación de Wagner tras aplicar el Heberprot-P
José Fernández Montequin1, Gabriela Mena2, Llipsy Santiesteban3, 1
1 Instituto de Angiología y Cirugía Vascular Calzada del Cerro # 1551, CP 12 000, Cerro, Ciudad de La Habana, Cuba
2 Hospital Militar Carlos Arvelo Caracas, Venezuela
3 Hospital Hermanos Ameijeiras Ciudad de La Habana, Cuba
ABSTRACT
To improve the quality of life of diabetic patients suffering from diabetic foot ulcers, and trying to diminish or avoid major amputations among them, we tested the use of Heberprot-P® (epidermal growth factor) in seventy of those patients having Wagner’s grade 5 diabetic foot. The product was administered by intralesional injection, three times a week. Twelve patients (17.1%) were amputated and seven (9%) abandoned the treatment. Otherwise, twenty eight patients (40%) saved their legs, and 23(32.8%) are currently being administered with 75 µg of Heberprot-P (EGF), with good results. Until this moment, Heberprot-P constitutes an answer for diabetic patients having diabetic foot grade 5 in the Wagner scale.
Keywords: Ischemic diabetic foot, human recombinant epidermal growth factor, diabetic foot Wagner 5, Wagner classification
RESUMEN
Para mejorar la calidad de vida de los pacientes diabéticos que presentan pie diabético, y con ello disminuir o eliminar amputaciones mayores en las piernas de estos pacientes, se ensayó el uso del Heberprot-P® (factor de crecimiento epidérmico) en 70 pacientes con pie diabético clasificado como grado 5 según la escala de Wagner. Se administró el producto por vía intralesional, tres veces a la semana. De los pacientes, 12 (17.1%) fueron amputados, 7 (10%) decidieron no recibir el tratamiento, 28 (40%) salvaron sus piernas al ser tratados, y en este momento hay 23 pacientes (32.8%) recibiendo el tratamiento con Heberprot-P de 75 µg con buenos pronósticos de salvar la extremidad. Hasta este momento, el Heberprot-P constituye una respuesta eficaz para los pacientes con pie diabético de grado 5 según la escala de Wagner.
Palabras clave: Pie diabético isquémico, factor de crecimiento epidermoide humano recombinante, pie diabético grado 5, clasificación de Wagner
INTRODUCTION
The discovery of growth factors opened a new era in the field of medicine for treatment of peripheral vascular diseases worldwide (1). In fact, this finding has benefited the outcome of diabetic foot, an entity responsible for 60% of major amputations being carried out among the population having peripheral vascular disorders (2). In this scenario, Heberprot-P (human recombinant Epidermal Growth Factor) irrupted in the international market in 1999 as a unique product to treat both neuropathic and ischemic diabetic foot ulcers (3). There are statistical reports on the appearance of up to 85% of total granulation in patients carrying severe grades of the disease (grade 1 to 4 of Wagner classification) (4, 5).
There are increasing evidences in different health services from several countries on using this growth factor. This product has proven efficacious in clinical studies for treating lesions of varying grade of the Wagner scale and wider than 20 cm2 (6, 7), clearing the therapeutic doubts about its efficacy, which are the common preconditioning for medicaments being tested in this pathology. Nevertheless, there remained concerns about which would be the effect of Heberprot-P when facing Wagner 5 diabetic foot lesions, a type of lesion so severe that most of the vascular surgeons do not hesitate to indicate limb amputation in almost 100% of patients.
The Wagner classification criteria establishes the grade 5 diabetic foot as that covered by extensive in the entire forefoot or the calcaneus area, also indicating the amputation of the affected limb (8). If we add ischemia to such an anatomically complex wound, the medical situation becomes more serious for the patient and his /her family and even more difficult the medical decision to take.
Even when the main recommendations made for Heberprot-P were established for grade 1 to 4 lesions based on efficacy, we also had the opportunity to challenge it at the Diabetic Foot service of the Carvelo hospital in Caracas, Venezuela, in all the range of grades. It is quite surprising the severity of the cases admitted in that institution because of being a terminal hospital (9).
The purpose of this report was to review the results obtained while applying the indicated dosage of Heberprot-P in patients carrying very advanced grade 5 lesions in the Wagner scale, with or without ischemia and with an amputation prognosis.
HEBERPROT-P ALLOWED TO SAVE WAGNER GRADE DIABETIC FOOT PATIENTS FROM AMPUTATION
From August 2008 to November 2009, up to 760 patients were admitted at the Program for Integral Attention of Diabetic Foot Patients (10), at the diabetic foot service in the Carlos Arvelo Military hospital in Caracas, Venezuela. Lesions were classified as ischemic or neuropathic after clinical and radiological examination, Doppler and angiographic tests (as required). All the patients showed some degree of local infection, as demonstrated by cultures and antibiograms, in serial analyses mostly. Following these, they were classified from grades 1 to 5 according to the Wagner scale for follow up.
The patients were assisted daily or in alternate days, as required by the lesion. Patients were admitted when needing antibiotic treatment and the established medical-surgical procedures were provided following the service’s procedures.
All the clinical inspections were done by an angiologist-vascular surgeon specialist, together with surgery interns, endocrinologists and nursing specialists. All the patients were subjected to sanitary-dietarydrug and metabolic control procedures as established for the Program, prior to administering Heberprot-P. The growth factor was injected in 75 µg doses in alternate days, until achieving local useful granulation tissue. Patients received the appropriate dressings with debridement when needed.
The dressing procedure included cleaning the lesion area with antiseptic solutions and further Heberprot-P application as recommended by the manufacturer (9).
Among the 760 patients treated, 70 patients (9.0%) carried a lesion classified as grade 5 in the Wagner scale. The anatomical distribution of the lesion varied, the most severe affecting the entire forefoot or the calcaneus areas, or both in some cases.
Twelve (17.1%) of those seventy patients were amputated due to the lack of a response to treatment as expected. Seven patients (10%) abandoned the treatment for different personal reasons.
A high impact result was achieved in 28 patients (40%) who had being indicated for amputation in other surgical services, all these patients discharged with complete granulation and in the epithelialization phase.
More significantly, the 23 patients (32.8%) still under treatment show a viable limb, although three of them still show signs indicative of amputation risk.
Considering all these data, we can conclude that up to 51 (72.8%) among the 70 Wagner grade 5 diabetic foot patients treated, for the best case scenario, could save their limbs which were formerly indicated for major amputation.
The average number of Heberprot-P doses was 14.5 among the 28 patients discharged. The lesion area covered a size range from 16 to 670 cm2. Fifteen (53.5%) of them required hospitalization for ten-day periods, with ambulatory follow up. Only 10 (43.4%) out of the 23 patients still under treatment have required a similar procedure.
CONCLUSIONS
These are very promising results for the specialists working in the medical-surgical care of the diabetic foot. They indicate that there is an efficacious therapeutic alternative for patients carrying a Wagner grade 5 diabetic foot ulcer, able to be considered prior to indicate a major amputation. The satisfactory outcome in 40% of the patients who were discharged completely healed sends a warning to surgeons on how much can be done before considering such an extreme exeretic procedure as limb amputation is. And the relative success could also increase in the near future to near 70%. There is no available therapeutic alternative providing such results.
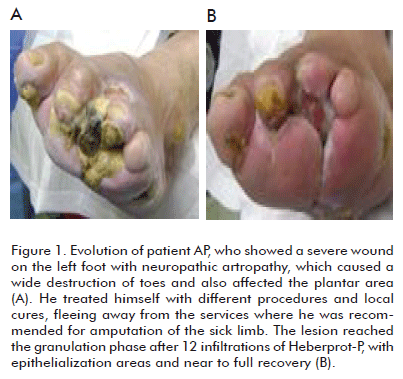
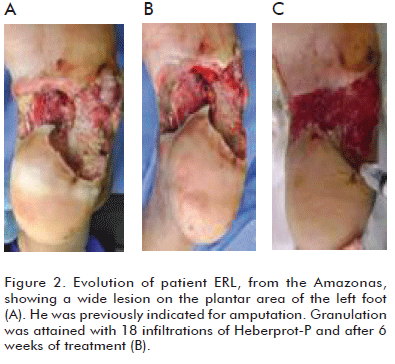
Can we assert that major amputations rates can indeed be decreased in Wagner grade 5 diabetic foot patients by administering Heberprot-P? The answer could be YES, if we establish an individual procedure for each patient, together with multidisciplinary teamwork and the efficient use of this product within the adequate clinical context, for a satisfactory recovery of the patient, saving the limb from amputation (Figures 1, 2, 3, 4).
REFERENCES
1. Berlanga J, Moreira E. Wound healing promotion in rats treated with EGF is dose dependent. Biotecnol Apl 1996;13 (3);181-5.
2. Sage RA, Pinzur, M. Amputation and Rehabilitation of Diabetic Foot. The Diabetic Foot, 2nd Edition. Edited por Logerfo & Velves.
3. Fernández-Montequín JI, Infante-Cristiá E, Valenzuela-Silva C, Franco-Pérez N, Savigne-Gutierrez W, Artaza-Sanz H, et al. Intralesional injections of Citoprot-P (recombinant human epidermal growth factor) in advanced diabetic foot ulcers with risk of amputation. Int Wound J 2007;4(4):333-43.
4. Acosta JB, Savigne W, Valdez C, Franco N, Alba JS, del Rio A, et al. Epidermal growth factor intralesional infiltrations can prevent amputation in patients with advanced diabetic foot wounds. Int Wound J 2006;3(3):232-9.
5. Fernández-Montequín JI, Valenzuela- Silva CM, Díaz OG, Savigne W, Sancho- Soutelo N, Rivero-Fernández F, et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: multicenter, randomised, placebo-controlled, double-blind study. Int Wound J 2009;6(6):432-43.
6. Fernández-Montequín JI, Betancourt BY, Leyva-Gonzalez G, Mola EL, Galán-Naranjo K, Ramírez-Navas M, et al: Intralesional administration of epidermal growth factor-based formulation (Heberprot-P) in chronic diabetic foot ulcer: treatment up to complete wound closure. Int Wound J 2009;6(1):67-72.
7. Fernández Montequin J, Santiesteban L. Experiencias en el uso del Heberprot-P en Venezuela. Reportes de casos del hospital Carlos Arvelo, Caracas. En: Infiltración del Factor de Crecimiento Epidérmico. Un tratamiento eficaz para la úlcera del pie diabético. Editorial Elfos Scientiae, 2009. p. 57-72.
8. Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, et al. Diabetic foot disorders: A clinical practice guidelines (2006 revision). J Foot Ankle Surg 2006:39(Suppl 5):1-66.
9. De Valera, L. Programa de Atención Integral al Paciente portador de Pie Diabético. Ministerio del Poder Popular para la Salud. Venezuela, 2009.
10. Mena G. Resultados de la aplicación de un Programa de Atención Integral al paciente portador del PD, con inclusión del tratamiento con Heberprot-P. En: Hospital Militar Carlos Arvelo, Coloquios Anuales, Abril 2008. Caracas, Venezuela.
Received in August, 2010.
Accepted for publication in September, 2010.













