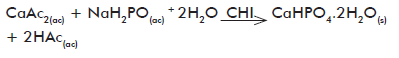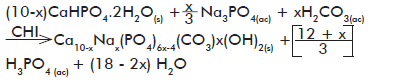Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl v.27 n.3 La Habana jul.-sep. 2010
REVIEW
Chitosan/hydroxyapatite-based composites
Materiales compuestos de quitosana e hidroxiapatita
Carlos Peniche1, Yaimara Solís1, Natalia Davidenko1,2, Raúl García3
1 Centro de Biomateriales, Universidad de la Habana, UH Ave. Universidad e/ G y Ronda, AP 6120, CP 10 600, Ciudad de La Habana, Cuba
2 Department of Materials Science and Metallurgy, University of Cambridge, Cambridge CB2 3QZ, UK
3 Instituto de Cerámica y Vidrio, Consejo Superior de Investigaciones Científicas, CSIC Madrid 28049, España
ABSTRACT
In the past 20 years, there has been a growing trend towards the development and use of biomaterials for the repairing and restoration of damaged bone tissue. The calcium phosphate bioceramics have attracted great attention in the field of orthopaedics because of its similarity to the mineral component of bone tissue. They have been used as granules for example in non-load bearing small implants, such as middle ear implants, in coatings on metals as dental implants, as well as in porous implants to stimulate bone growth within the implant; and cements, which are implanted in a paste like form and harden in vivo. The clinical disadvantages associated with them are primarily focused on poor mechanical strength and slow resorption kinetics as compared with the surrounding tissue. Therefore, current studies are aimed at creating new formulations combining calcium phosphate compounds with biopolymers, in order to avoid the frequent migration of bioceramic particles from the implant site, reducing potential damage to soft tissue in the vicinity of the implant and to improve biodegradability, curing properties, mechanical strength and injectability. The aims of this paper is to bring a review of the literature concerning chitosan-hydroxyapatite composites for bone restoration, and to mention the main methods of preparation, physico-chemical and biological properties, and tissue engineering techniques using these materials.
Keywords: composite, hydroxyapatite, chitosan, bone tissue, bone tissue engineering
RESUMEN
En los últimos 20 años se observa una tendencia creciente del desarrollo y empleo de biomateriales para la reparación y regeneración del tejido óseo dañado. Las biocerámicas de fosfatos de calcio han despertado gran interés en el campo de la ortopedia debido a su similitud con el componente mineral del tejido óseo. Estas se han utilizado como gránulos en implantes pequeños que no tengan que soportar cargas, como el oído medio; en recubrimientos sobre metales que las refuercen, como los implantes dentales, los implantes porosos para estimular el crecimiento de un hueso dentro del implante, y los cementos que se implantan en estado pastoso y fraguan in vivo. Los inconvenientes clínicos asociados con las biocerámicas se centran fundamentalmente en la pobre resistencia mecánica y la lenta cinética de reabsorción en comparación con el tejido circundante. Es por ello que los estudios actuales están encaminados a crear nuevas formulaciones compuestas por fosfatos de calcio y biopolímeros, con vistas a evitar la migración frecuente de las partículas biocerámicas del sitio del implante, disminuir la posibilidad de daños a los tejidos blandos próximos al implante, mejorar la biodegradabilidad, las propiedades de fraguado, la resistencia mecánica y la inyectabilidad de los biomateriales. Los objetivo/s de este artículo son ofrecer una revisión de la información actualizada sobre materiales compuestos (composites) de hidroxiapatita y quitosana como sistemas soporte del tejido óseo; mencionar los principales métodos de preparación, las propiedades físico-químicas y biológicas, y las técnicas de ingeniería de tejidos que utilizan estos materiales.
Palabras clave: composite, hidroxiapatita, quitosana, tejido óseo, ingeniería de tejido óseo
INTRODUCTION
Nowadays, bone infections are among the main problems to be solved in regenerative medicine, due to the increase in people longevity and the high incidence of accidental traumas. According to the World Health Organization (WHO), osteoporosis is the second sanitary assistance health problem worldwide, after the cardiovascular diseases. For instance, up to 60 000 hip fractures are annually reported in Spain, accounting for 20 to 22% of the hospitalization capacity at Orthopedic and Traumatology Services (1).
There is a high prevalence of osteoporosis in Cuba, with a relatively early average onset (before 65 years). More than three quarters of the cases suffer from moderate to severe osteoporosis with a 19% frequency of fractures. In fact, mortality rates are rapidly increasing among the elderly population due to combined incidence of a hip fracture-associated falling and osteoporosis (2). For example, only in Matanzas province 609 deaths of hip fracture from 2001 to 2007 have been reported. Mortality increased from 1.6 to 2.0% during that period, and the hip fracture/accident death ratio subsequently rose from 32.2 to 46.1. Moreover, the mortality rate of hip fracture proportionally correlated with age increase and gender; the amount of women affected almost doubled the number of men (16.4 vs 9.5)(3). Currently, osteoporosis has been called the "silent epidemics of the XXI century", because of being the main cause of bone fracture hmong post-menopausal women and in elderly people (4). Its affections are not fatal but can seriously deteriorate the patient´s quality of life by provoking clinical manifestations of chronic pain, physical disability and esthetic problems.
An alternative to these troubles consists on using bone substitutes, either to replace or to favor the regeneration of the affected tissue. In such cases, bone tissue extracted from the same patient is the most common alternative favoring the best recovery. However, there is a finite amount of bone available to be selftransplanted, also requiring a second surgical intervention. In spite of its limitations, materials used as bone substitutes have included autogenous bone (demineralized bone matrix), metals (titanium, stainless steel and alloys), polymers (methyl polyacrylate), polyesthers (e.g., polylactic and polyglycolic acids), ceramics (alum, calcium phosphates such as hydroxyapatite (HAp), tricalcium and octocalcium phosphate, and silica-based as bioglass and silica-doped nanoapatite), tricalcium b-phosphate (b-TCP) macroporous implants and composites of natural or synthetic HAp with polymers, sometimes combined with biological substances for a more appropriate presentation (5). Research is increasingly focusing on the design and development of new materials able to stimulate regeneration and repair of damaged bone tissues, aiding a fast recovery of the patient and lowering the high costs of surgery and materials available in the international market.
In Cuba, a natural origin HAp was developed (Coralina, HAP-200), which has been applied successfully as bone graft substitute and for integrated ocular implants (6). There is also a synthetic HAp (Apafi l-G), being used in bone defect implants with good results (5). Other HAp- and porogenic additivesbased calcium phosphate cements (CPCs) were also developed.
Nevertheless, as referred in the specialized literature, there has been a significant trend during the last years on the development of ceramics- and polymerbased scaffolds for tissue engineering. This is due to the excellent osteoinductive properties of ceramic materials, instead of its low degradation, low mechanical resistance and hard to be mold to fit the physical and geometric requirements of the given site or bone defect. At the same time, biopolymers bear low osteoinductivity but with better mechanical and degradation properties. Thus, biopolymer-based calcium phosphate composites allow incorporating advantageous properties of both types of components (7).
Chitosan (CS) is one of the components most frequently used to prepare calcium phosphate composites, because of its biocompatibility, biodegradation and innocuousness. Due to the interest on composites based on this biopolymer, here we review the state of the art and current trends using CS-based calcium phosphate composites, and especially HAp.
CALCIUM PHOSPHATES
Calcium phosphates have been demonstrated to be the ideal biomaterials for bone implantation during the last 30 years, because of being biocompatible, bioactive and bone-conductive. The development of calcium phosphate-based bioceramics for clinical application began in the 1970s, due to the failure of other previously used materials such as: steel, cobalt alloys and poly (methyl polyacrylate).
The study of calcium phosphate-based ceramics has focused on composites of the tertiary system CaOP2O5-H2O, mainly hydroxyapatite (Ca10(PO4)6(OH)2) and tricalcium phosphate (Ca3(PO4)2), due to its increased bioactive potential shown in several investigations (8-10).
Among them is Coralina. This is a biomaterial obtained from white corals of the Porites porites family which are very common in the Cuban insular shelf, and which bears a crosslinked tridimensional structure quite similar to the human bone trabecula. It is produced as big blocks to fill cavities and in small pieces to replace bone fragments. More than 20 000 patients have been reported as receiving implants of porous HAp with a 97% of bone integration. Only 3.2% of those implants were considered as failures, mainly due to postoperative immediate sepsis requiring biomaterial removal, tumor relapse, and mobility of implants with poor osteosynthesis, and other causes but not to the implanted biomaterial. Coralina application in these specialties allowed treatment and correction of endobucal and facial bone defects, with excellent aesthetic and clinical results (6).
There is also Apafi l, a ceramic granulate of highly pure and dense synthetic HAp of different size granules. It has been successfully applied as filling material for periodontal and periapical defects of pathological or traumatic origin, in cystic cavities, to fix intra-osseous dental implants, for filling of autogenous dental transplants cavities and alveolar cavities, to remodel the alveolar edge and to recover the dental pulp, and in rehabilitating tooth root lesions and craniotomies. The preclinical and clinical experiences have demonstrated that Apafi l-based HAp ceramics are biocompatible, bioactive, biologically stable and boneconductive (11, 12).
Bioceramics are only available as blocks or granules, preconditioning some limitations. Granules, for example, are used in small implants, in non-load bearing areas as the middle ear, or in dental implants as coating for the metals used as reinforcement, since bioceramics are rigid and fragile. But it has been proven that these granulated materials are able to migrate to the neighboring sites, increasing the frequency of complications or even causing surgery failure. At the same time, block bioceramics not always fit properly to the treated defect (13-15), and in those few cases where a proper adaptation of the implant is achieved, there is a potential risk of failure due to its very slow resorption kinetics and poor mechanical strength which could result in a short-to-long term fracture.
According to these reasons, partially bioresorbable alternatives have been explored, more similar to the bone tissue to be replaced and serving as scaffold for osteogenesis at the same time that implant resorption occurs. In this sense, partially resorbable ceramics of b-TCP have been developed, obtaining macroporous granules by using an oxygen peroxide solution and yeast powder as porogenic agents (16).
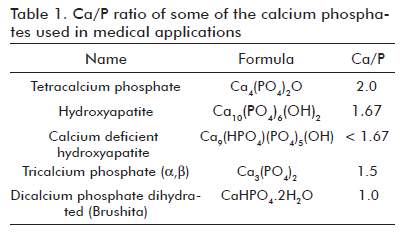
Nonetheless, the bone natural mineral component is mainly non-stoichiometric HAp (of Ca/P ratio other than 1.67) which differs from the stoichiometric HAp not only in the chemical composition but also in crystallinity and the specific surface area, these two properties making it more reactive in a biological environment. Some authors mentioned that HAp biodegradation inversely correlates with the Ca/P ratio. In other words, the calcium-deficient HAp is more biodegradable than the stoichiometric HAp due to a low crystallinity (17). The Ca/P ratios of some calcium phosphates used for biomedical applications are shown in table 1.
It was recently reported the development of a calcium- deficient HAp (Ca/P ratio lower than 1.67) of nano-structured morphology, which was doped with silicate ions to imitate the biological apatite, which was able to increase the solubility and resorption capacity in vivo of the composite materials it was designed for, also increasing their bone-conductivity and bioactivity (18).
Simultaneously, a whole range of possibilities arose with the discovery of CPCs in the 1980s for using calcium phosphates in bone tissue regenerative applications. In general, CPCs are composed of two phases, one solid and one liquid, which form a paste at mixing and harden under physiological conditions once implanted by injection. For this reason, they can be used in low invasive surgical procedures.
Some recent studies focused on creating acrylic bone cements modified with HAp micro and nanoparticles, more resistant to compression and traction than the same type of materials used for more than 40 years in orthopedics to fix the articular prosthesis to the bone (19).
Another current trend for preparing bone implant calcium phosphates comprises adding macroporosity. This relevant property supports the fast cellular colonization of the implanted material (Figure 1) by accelerating the vascularization process and, therefore, a satisfactory repair of the bone defect (20, 21), this property having to be tightly controlled for the bioresorption of the bone implant. Some authors consider as macroporous materials those having pore diameters from 50 to 250 mm. However, Kawachi et al. advise that the ideal pore diameter must be above 100 mm for bioceramics (22).
The methods used for pore formation can be divided in two major groups: emulsion formation, to generate pores during the hardening process but followed by a thermal procedure to achieve the required porosity, and the addition of highly soluble and non-toxic crystalline substances, adequate to produce the required porosity during powder mixing. This last method requires dissolving the additives once the cement has been applied and hardened onto the bone defect.
The use of mannitol, sucrose, and frozen particles of a sodium phosphate solution containing surfactant molecules are the most established techniques to induce porosity in CPCs. All of them support the formation of macropores on CPCs, but of deficient crosslinking. To solve this problem, some authors have implemented pore formation by in situ gas formation because of adding CaCO3 to the cement powder, followed by a reaction while mixing (23). Recently, a technique was developed for macropore formation in CPCs by using egg albumin as foamy agent during cement hardening (16). The same authors ran several experiments to obtain macroporous HAp scaffolds from CPCs with the aid of hydrogen peroxide as foamy agent, producing cements with crosslinked macroporosity of pore wider than 200 mm, the best report on CPCs so far (24).
Nevertheless, it has been demonstrated that CPCs still have a very slow resorption kinetic and a poor mechanical strength in spite of its described advantageous properties, both considered disadvantageous due to the weakness of the material as compared to the surrounding tissue (25). These limitations have led to investigate on biopolymer-based calcium phosphate composites, bearing properties which can be incorporated to the resulting material.
BIOPOLYMERS
Polymeric materials are widely applied in the field of medical implantology, due to their similarity to the physical-chemical properties of the living tissues which are mainly composed of natural polymers or biopolymers such as proteins and polysaccharides (26). These materials have been used for drug controlled release and tissue engineering, because of being biocompatible, non-toxic and biodegradable at physiological conditions, and showing a variable in vivo resorption kinetic according to the properties of the given macromolecule.
As mentioned above, there is a current trend on using biopolymers to formulate materials for periodontal, maxillofacial and bone tissue engineering applications. The physical properties of the polymeric materials are similar to those of the organic phase present in bone tissues, in spite of having certain limitations hampering its application in those body structures subjected to high and cyclic loads and tensions, such as hip and knees. These properties allow establishing bone structures through their matrices, supporting the fastest osteointegration, and resorption of natural polymer composite materials.
Collagen, jelly, alginate, chitin and CS are among the most widely studied biopolymers. CS has been extensively reported as a versatile biopolymer, due to its excellent properties and diverse natural sources, being considered by some groups as the natural polymer of the 21st century and with several applications in biotechnology, biomedicine and recently in tissue engineering (27).
CHITOSAN
Chitosan ((1®4)-2-amino-2-desoxy-b-D-glucan) (Figure 2) is industrially produced by extensive deacetylation of chitin ((1®4)-2-acetamide-2-desoxy-b-Dglucan), a polysaccharide widely found in the animal kingdom and the second in abundance after cellulose what makes it an important renewable resource.
Chitosan is composed of two types of structural units randomly distributed (Bernoulli distribution) along the chain: N-acetyl-D-glucosamine (A) and Dglucosamine (D), linked together by glycosidic type b(1®4) bonds. In its crystalline form, CS is normally insoluble in neutral water solutions, but soluble in diluted acidic solutions (pH< 6.0) where glucosamine amino free groups become protonated (28).
Depending on the source and preparation procedure, CS molecular weight can range from 300 to 1000 kDa with a deacetylation degree (DD) of 60 to 95%. Chitosan has been demonstrated to be biocompatible and biodegradable, because of promoting cell adhesion and being reabsorbed by means of hydrolysis by enzymes present in the physiological fluids (29, 30).
It can be considered a structural analog of glycosaminoglycans (GAG)(31, 32), anionic polysaccharides generally bound to proteins by electrostatic interactions to form proteoglycans. The last ones play a relevant function in organizing and functioning of the extracellular matrix in human tissues (33).
Chitosan- and GAG-based materials, said CS and chondroitin sulphate, have shown adequate cytocompatibylity for bone regeneration, successfully used for cartilage repair and as artificial skin components to treat dermal lesions. Studies comparing different materials composed of CS and alginate, or polylactic acid (PLA), for articular tissue engineering, showed that CS could increase adhesion and cell proliferation in vitro and support a better biosynthetic activity than alginate and PLA (34).
Lee et al. (35) obtained a scaffold formulated with CS, GAG, collagen and loaded with tumor growth factor b1 (TGF-b) for its controlled release to promote articular regeneration. They improved the mechanical properties and stability of the collagen macromolecules´ crosslinking by adding CS, also inhibiting the action of collagenases. Aimin et al. (36) demonstrated the antibacterial activity of CS, a subject extensively referenced in the consulted literature (37). They evaluated CS in experimentally-induced osteomyelitis, showing a reduction in the spread of a Staphylococcus aureus infection. Sano et al. (38) studied the influence of CS DD and molecular weight on the inhibition of Streptococcus sobrinus 6715 in saliva treated with hydroxyapatite, and demonstrated the potential antiplatelet effect of high molecular weight CS (5-6 kDa) and intermediate DD (50-60%) (39).
Chitosan biodegradability is another essential factor to consider when selecting biopolymers for tissue engineering. Lysozyme is the enzyme present in biological fluids responsible for CS degradation in vivo.
Lysozyme hydrolyzes the b-1,4 de N-acetylglucosamine bonds close to a glucosamine residue. By these means, lysozyme action on high DD CS (> 80%) is weak, with no significant changes in the molecular weight of the hydrolyzed CS over time. This results in long polymer degradation periods, delaying in months the in vivo reabsorption of the CS-based material (40). Mao et al. (41) observed a direct correlation between CS DD and cell adhesion. A fast biodegradation could be achieved by using low DD CS, but limiting cell adhesion. Therefore, the adequate selection of DD for the CS to be used is critical to develop tridimensional scaffolds and control its biodegradation and biocompatibility.
The abovementioned properties of CS (42), together with its demonstrated healing and hemostatic properties, its plasticity for presentation (microspheres, membranes and bidimensional films, or in tridimensional scaffolds), flexibility, mucoadhesivity and wettability, also its facility to promote bone formation by cellular osteogenesis at the required site, among other desired properties (43), support using CS as an excellent polymeric candidate in orthopedical applications, especially for bone tissue engineering (44). Nevertheless, CS is not bioactive as biopolymer and show poor mechanical properties.
All these explains the growing interest on combining the bioactivity and biodegradability of inorganic materials together with the rest of CS properties to obtain new compound materials (composites) of improved mechanical characteristics also favorable for bone tissue engineering (45).
PREPARATION OF CS AND HAP COMPOSITES: MAIN FORMULATIONS, PROCEDURES TO OBTAIN THEM AND APPLICATIONS
Calcium phosphate composites with CS matrices were extensively investigated in the last decade for structural purposes, specifically for orthopedics and maxillofacial surgery (46), either to fill bone defects, to increase the alveolar edge, middle ear implants, fusion of spine vertebrae or to coat metallic prosthesis, due to a proper filling-matrix integration (47). Those hybrid materials have been prepared by different methods, as shown in table 2.
CHITOSAN AND HAP SIMPLE MIXING METHOD
The method of simple Hap mixing with CS is one of the most common methods used to prepare composites, especially tridimensional scaffolds for tissue engineering (48). The technique consists in mixing the previously prepared ceramic powder with a CS solution to generate a suspension as homogeneous as possible. Scaffolds are generated from this mix as porous sponges by freezing and lyophilization (49, 50). Murugan et al. (51), for example, used this approach to develop a simple procedure, by using the wet way, to generate nanostructured compound systems (nanocomposites) of HAp and CS in two steps: HAp precipitation in alkaline medium (pH 10.0 with NH4OH) starting from CaCl2 and (NH4)2HPO4 as precursor salts and further mixing of nanometric HAp with solutions at different CS concentrations in acetic acid at a fixed temperature.
A similar procedure was used by Finisie et al. (52) to generate HAp and CS composites in pills. They started from a paste made up of a mix of HAp, aluminum and CS at different proportions. Due to aluminum toxicity, they transformed it onto sodium aluminate by using a concentrated solution of sodium hydroxide, resulting in the formation of pores wider than 100 mm which were generated by the hydrogen gas released by the reaction.
INCORPORATION OF CS TO CPCS
The incorporation of CS to CPCs (Figure 3) is another very promising way to obtain composite materials, since it improves injectability, the degradation rate, hardening properties and mechanical performance of the resulting materials (53). In this case, a suspension of the solid fraction is mostly obtained in the polymeric matrix with pH near 6 where there is a fraction of CS protonated amino groups enough for solubilization, aiding to include CS into the liquid fraction of the cement. Afterwards, an injectable viscoelastic paste is prepared, which contains CS incorporated in the liquid fraction of the cement and the solid fraction HAp precursors. Once injected, the paste hardens due to the rise of pH to physiological 7.4 and the subsequent neutralization of the amino groups. In this process, the inorganic load becomes physically entrapped (in situ hardening) within the gel matrix in 5 to 8 min, an interval shorter than for control CPCs (61.7 min.) which are devoid of CS (54). The resulting materials also show improved mechanical properties, as resistance to compression of 15 to 25 MPa, higher than the same parameter for the CPC alone (10.4 MPa) (55).

Taking advantage of these facilities, some investigations have focused on CS-CPC composites (56-62) as materials with potential application in bone tissue engineering and for controlled release of therapeutic agents, showing very promising results in periodontal bone defect regeneration (63). In this sense, a vast experience has accumulated on the synthesis and characterization of compound materials by using calcium phosphates as filling materials (b-TCP and HAp, treated superficially or not), natural polymers (CS, sodium alginate), and acrylics (mainly poly acrylic acid, polyacrylamide and poly(methyl methacrylate), poly(2-hydroxyethyl methacrylate)) to restore bone tissue and as potential controlled release systems for active ingredients (64, 65)
COATING METHOD
Calcium phosphate composites can be obtained as coating by using electrochemical and electrophoretic procedures, which favor the deposition of HAp and CS particles. This technique commonly known as electrochemical deposition allows preparing hybrid materials at room temperature, which is highly favorable considering the final biomedical application.
Recently, Pang et al. (66) developed a method of layered electrophoretic co-deposition of the CS-HAp composite for protecting steel-made materials getting into contact with physiological solutions. To modify the surface of shape memory materials made up of nickel- titanium alloys and protect them from corrosion, Sun et al. (67) prepared composites of heparin, HAp and bioglass in a CS polymeric matrix as films, by cathodic electrophoretic deposition. Redepenning et al. (69) also prepared HAp and CS composites by an electrochemical method, starting from brushita and CS in alkaline medium, to coat titanium materials (68).
Additionally, some authors have studied the remarkable properties of this technique by using biomaterials as Biovetir II, coated with nanostructured CSHAp composites to improve the implant interaction with cells and its fixation to the subcutaneous tissue (69), as well as for middle ear reconstruction (70).
IN SITU PRECIPITATION METHOD
In spite of the overwhelming advantages of the abovementioned methods, it has been demonstrated that compound systems prepared by mixing techniques, which incorporates CS into CPC, and those coating HAp particles with CS solutions are frequently nonhomogeneous at microscopic level because of the hard to obtain uniform distribution of the inorganic components in the womb of the polymeric matrix. These can generate practical inconveniences, since the lack of homogeneity can induce inflammatory symptoms by forming voluminous fibrotic capsules of dense or granulated connective tissue. This originates a strong rejection of the implanted material or changes in the area surrounding the prosthesis.
In that sense, the method of in situ precipitation of the bioactive inorganic component inside the polymeric matrix, as referred in several publications as biomimetic or bio-inspired method (71) could be more attractive to prepare this type of composites by permitting to generate much more homogeneous systems.
This procedure is nature-inspired in bone, dentin, egg and mollusk shells, etc., showing a perfect combination of the structure and composition characteristics responsible for the micro and macro-properties. In situ precipitation of calcium phosphates allows controlling the internal architecture (structure) and the chemical composition of the resulting materials.
This procedure is based on obtaining nanostructured and crystalline composites of the biologically active component (calcium phosphate), homogeneously disperse in the polymeric matrix (CS) to mimic the biological apatite of the bone tissue. This supports its application in bone tissue engineering, specifically in targeted bone regeneration (72), and controlled release of drugs (73) and biologically active molecules (74).
These materials have being traditionally prepared by several methods. Yamaguchi et al. (75) have developed a single-step co-precipitation methodology by dripping CS in H3PO
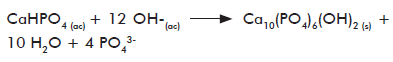
The same group suggested the use of salts and weak acids as precursors of calcium and phosphate ions, instead of strong acids or bases to avoid their potential destructive effect on the polymeric chains of CS during composite preparation. Chang et al. (80) reported a similar procedure to prepare HAp and gelatine nanocomposites, with Ca2+ and PO43- ions concentrations controlled by diffusion, through dissolution and co-precipitation processes.
These in situ production methods have supported an adequate incorporation of inorganic fillings inside composites´ structures, either at micro or nanometric scales. Noteworthy, each of the methods referred generates a particular type of CS and HAp composite material, with specific structural properties.
Considering that the mineral phase of the bone is composed of non-stoichiometric HAp (Ca/P ratio < 1.67), it is convenient to simulate the most biological apatite with the inorganic component on these composites. For this purpose, Peña et al. (81) mixed brushita powder (CaHPO4 2H2O) with a CS citric acid solution, and keeping the solution alkaline by adding sodium hydroxide. The use of citric acid dissolutions as solvent is questionable in spite of the similarity of the apatite phase to that of the final composite, since it inhibits HAp transformation while nucleating amorphous calcium phosphates. Besides, there was also mentioned in the literature that citrate ligands can affect crystal and cell size because of including carboxyl groups (82).
We have prepared in our lab composites of CS and calcium-deficient HAp starting from acetic acid solutions of HAp precursor salts (calcium acetate and NaH2PO4H
Optical micrographs as those shown in figure 4 correspond to the inorganic phase crystals obtained on each phase according to equations 2 and 3, prior to (calcium hydrogen phosphate; CaHPO4·2H2O or DCPD) and after (calcium-deficient HAp precipitated in the absence and presence of CS) the hydrolysis with Na3PO4.
A morphological examination shows that in the first step, the inorganic phase precipitating under the reaction conditions used corresponds to the typical flattened crystals of brushita (CaHPO4·2H2O). However, after hydrolysis with Na3PO4, much smaller crystals were obtained by formation of the expected apatite phase (calcium-deficient HAp), achieving a complete transformation of one crystalline phase into the other.
We have recently prepared via in situ composites of CS with non-stoichiometric HAp incorporating silicate ions to generate a material of higher osteointegration to the biological system (84). The load of antibiotics and other active principles (for example, growth factors) into these types of tridimensional matrices could be a way to increase cell proliferation, with faster and more efficient formation and mineralization of the bone tissue.
PHYSICAL-CHEMICAL CHARACTERIZATION OF COMPOSITE SYSTEMS OF CS AND HAP
The physical-chemical properties of CS and HAp are highly similar to those of the bone tissue (85). The biodegradable, biocompatible and bone-conductive performance of these composite materials is very relevant for future medical applications (86).
In general, the inorganic reinforcement of this type of composites should provide simultaneously bioactivity together with resistance and rigidity to the resulting material, the polymeric matrix functioning as filling. However, it has been demonstrated that the properties of the generated material, the biological properties included, can be improved by incorporating CS (87).
BIODEGRADATION
Biodegradation or the changes affecting the implanted device when exposed to the natural physiological or simulated medium is critical and has been extensively addressed in the literature (88, 89). Several factors can be involved: physical-chemical dissolution, physical breakdown of bigger molecules into smaller ones by biological agents as enzymes (90) what decreases pH at the site (91), or the properties of the polymeric matrix used, among others (92, 93).
In the same line of evidence, Yang et al. demonstrated that the degradation kinetics can be controlled by selecting the N-acetylated CS derivative, also accelerating its resorption (with lower DD values) due to the preferential action of lysozyme on the glycosidic bonds between the acetylated units of the biopolymer (93). Nevertheless, Freier et al. observed that lower DD CS films induce poor cell adhesion (88). Similarly, Chatelet et al. showed that fibroblasts did not proliferate on CS films regardless of its DD value, due to a tight anchoring of these cells onto the material that prevents its further proliferation (94). Putting all together, it suggests that biological properties of CSbased composite materials not only depend on the acetylation/ deacetylation balance of the natural polymer, but also on the cell type used to promote adhesion and proliferation on the given substrate.
A method to evaluate the degradation rate comprises assessing the release of Ca2+ ions from composites submerged into a physiological medium. Results show an increasingly faster degradation rate by increasing composites CS content. The hydrophilic nature of the CS polymeric matrix generates a low crystallinity in composites, subsequently rising the solubility of the system, the dissolution speed and the release of calcium ions to the medium. This supports a gradual rise of bioresorption resulting from the increased biopolymer content within the composites (95).
MORPHOLOGY
The final morphology of composites is another significant property influencing the functionality of these materials. It is affected by reaction conditions, such as: concentration, temperature, pH, 3D conformation, time and filling-matrix interaction.
Generally, composite morphology can widely vary depending on the geometric versatility provided by the polymeric matrix (films, micro and nanoparticles, membranes, cements, etc.) and the intended application. Normally, morphology, size and distribution are measured by optical and/or scanning and transmission electron microscopy techniques. A relevant aspect is the apatite phase obtained on the surface of the composites, which frequently appears as globular aggregates or clusters of tiny crystals with different characteristics depending on the predominant inorganic component in the given composite (96). For example, b-TCP is presented as prism-like particles, the octocalcium phosphate as small clustered plaques, quite similar to the non-stoichiometric HAp, and DCPD as wide, flattened crystals of great size, and calcium deficient HAp generally appearing as thin and small needles projected from a central point (Figure 4) (97).
COMPOSITION
Composition of CS/HAp is frequently studied by infrared spectroscopy to identify the main functional groups and the presence or absence of carbonate ions. Crystallinity and the inorganic phases present are assessed by X-ray diffraction, and Ca and P content determined by energy dispersive spectroscopy, while the composition of the system is evaluated by thermogravimetry and differential thermal analysis (98).
The individual components of CS calcium phosphate composites show a stable physical-chemical identity; in other words, they are neither dissolved nor completely fused one into the other. Thus, there can be independently identified the inorganic phase and the polymeric matrix. Nevertheless, they can interact through the reactive amino groups on CS and the phosphate and hydroxyl groups and calcium ions on the HAp (99).
BIOLOGICAL ASSAYS OF CS/HAP COMPOSITES
Biocompatibility is the property of any material determining its fitness to a specific application without inducing allergic, immunitary or any other type of reaction when getting into contact with living tissues (100). Calcium phosphate and CS composites have shown to be biocompatible in biological assays with different cell types (101).
In another experimental setting, HAp composites based on CS and alginate and soaked with drugs showed effective as local delivery systems in vitro (102). Similarly, the in vitro encapsulation within CS/ HAp microspheres and bone-conductivity of human periodontal ligament fibroblasts were assessed, providing an adequate osteogenic differentiation (103).
Additionally, bioactivity is the ability to support the formation of a biologically active carboHAp layer onto its surface, facilitating the interface junction with the bone tissue and guaranteeing a close attachment to the bone without an interposed fibrotic capsule. This supports the efficient material-bone integration, allowing the presence of bone cells as required for a successful regeneration process on the material´s surface (104). Hence, the inorganic phase to be integrated should be chemically and structurally similar to the mineral phase of the bone. In this sense, the excellent bioactivity of HAp (105) and the presence of the Nacetylglycosamine in CS (GAG analog) stimulate bone growth (106).
Biodegradability is another relevant property for tissue engineering systems, also considered for its selection and with CS positively contributing to it. By these means, a dynamic process of formation and resorption of bone tissues is established, where these materials serve as scaffolds for tissue infiltration and replacement, simultaneously stimulating cell growth within (107).
Recently, porous tridimensional scaffolds of calcium phosphate and CS composites were generated for bone tissue engineering (108, 109). These systems, obtained from CPCs and CS derivatives, tricalcium phosphate/CS, alginate/HAp and CS/HAp (110) supported fast osteoblast differentiation and growth (Figure 1), with osteogenic effects (111). Zhao et al. (112) prepared a 3D lattice of HAp/CS/gelatin (a composite with a structure similar to that of the human bone) by in situ precipitation and intended for bone tissue engineering, to induce favorable adhesion, growth and osteogenic differentiation of cultured human mesenchymal cells.
Additionally, the group of Zhang et al. (113) has published several works where they obtained calcium/ CS porous scaffolds, alternatively incorporating bioglass for human MG63 osteoblast cell culture. Only osteoblast cells were able to proliferate or differentiate within all the composite materials (phenotypic proliferation). The use of CS scaffolds together with calcium phosphate bioglass avoid the fast degradation in the physiological medium, this process releasing acid byproducts which could affect the osteoblast differentiation (114).
Also Zhao et al. (115) developed hybrid biomimetic materials of HAp/CS/gelatin to analyze cell behavior in rats. Results showed cell proliferation, de novo formation of bone tissue and mineralization of the obtained scaffolds in only 3 weeks (116).
These investigations demonstrate the feasible use of calcium phosphate/CS tridimensional matrices as adequate systems for scaffolds and implants in bone tissue engineering procedures (117). The proper selection of the components for the system to be designed will be determined by the final application of the scaffold.
CONCLUSIONS
Ceramics of calcium phosphates as HAp, osseous CPCs and b-TCP, are biocompatible, bioactive and osteoconductive, these properties making them excellent materials for orthopedics. Nevertheless, their availability as granules or blocks, the mechanical weakness of CPCs and the slow resorption or degradation in vivo of all these materials limit their application and the useful lifespan of the material with adequate characteristics, both short and long term in the host organism. Composites formulations based on natural polymers as CS and the range of preparation methods available allowed obtaining materials of improved and fitted design, of increased strength and improved bone-conductivity, homogeneity, bioactivity, biodegradability and similar to the bone tissue to be repaired or replace. All these widen their application in the fields of orthopedics and traumatology, more than precedent materials. Current trends tend to develop composite tridimensional scaffolds of improved properties, starting from inorganic materials of higher biodegradability and bioactivity (for example, Si-doped non-stoichiometric HAp) and incorporating antibiotics and other active principles (e.g., growth factors) into the matrix of CS or other polymer to promote an adequate cell proliferation, and a faster and more efficient formation and mineralization of the injured bone tissue.
REFERENCES
1. Hardiman H. Osteoporosis: The lifelong risks. Practice Nursing 2006;17:328-33.
2. Ochoa F, Pereira O. Encuesta sobre osteoporosis en un área de salud. Rev Cubana Endocrinol 1997;8(2):135-41.
3. Rodríguez P, Fernández J, Díaz O, Garrote I, Morales JM, Achiong FJ. Fallecidos por fractura de cadera en la provincia de Matanzas. Rev Méd Electrón 2009; 31(5). Available in: http://scielo.sld.cu/scielo.php?pid=S1684-18242009000500001- &script=sci_arttext (Consulted: October 19th, 2009).
4. Jordán M, Pachón L, Ponce de León L, Robainas I, Moreno SE. Osteoporosis: ¿Un problema de salud prevenible? Rev Méd Electrón 2006;29(5). Available in: http://wwwcpimtz.sld.cu/revistamedica/año2006/tema16.htm (Consulted: October 19th, 2009).
5. Legrá R. Tratamiento de los defectos óseos mediante implante con Apafil-G. Tesis presentada para optar por el título de Especialista de Primer Grado en Periodoncia Universidad Médica de Guantánamo, Guantánamo, Cuba, 2003.
6. González R. Hidroxiapatita porosa Coralina Hap-200. 15 años de aplicaciones clínicas. Rev Cenic Cienc Biol 2005; 36 (Especial):1-8.
7. Mushipe MT, Revell PA, Shelton JC. Cancerous bone repair using bovine trabecular bone matrix particulates. Biomaterials 2002;23(2):365-70.
8. Bohner M. Calcium orthophosphates in medicine: form ceramics to calcium phosphate cements. Injury 2000;31:S-D3-47.
9. Orlovskii V, Barinov S. Hydroxyapatite and hydroxyapatite-matrix ceramics. J Inorg Chem 2001;46:S129-49.
10. Peón E, Fuentes G, Soares M, Galván JC, Llópiz JC, Bastos IN, et al. Hydroxyapatite coating by sol-gel on Ti-6Al-4V alloy as drug carrier. J Mater Sci Mater Med 2009;20:543-7.
11. Álvarez M, Carrodeguas RG, García- Menocal JA, Díaz IF, Argüelles DM, Hernández JR, et al. Implantation of cuban granulated hydroxyapatite Apafill-G in pericapical bone defects. Bioceramics 1998;11:583-6.
12. García D, García L, Pérez MC, Suárez M, Delgado JA, García R, et al. Filling of post-extraction dental socket with hydroxyaptite granules Apafil-GTM. Key Eng Mater 2001;192-5:925-8.
13. Liou SC, Chen SY. Transformation mechanism of different chemically precipitated apatitic precursors into betatricalcium phosphate upon calcinations. Biomaterials 2002;23:4541-7.
14. Vallet-Regí M. Ceramics for medical applications. J Chem Soc Dalton Trans 2001:97-108.
15. Liou S, Chen SY. Transformation mechanism of different chemically precipitated apatitic precursors into beta-tricalcium phosphate upon calcinations. Biomaterials 2002;23:4541-7.
16. La Serna AA. Desarrollo y caracterización de cementos óseos macroporosos de fosfato de calcio. Tesis presentada en opción al grado científico de Doctor en Ciencias Técnicas. Instituto Superior Politécnico José Antonio Echeverría, La Habana, Cuba, 2005.
17. Klein CPAT. Calcium phosphate implant materials and biodegradation. In: Academish Proefschrift. Vrije Universiteit te Ámsterdam; 1988.
18. Davidenko N, Carrodeguas RG, Peniche C, Solís Y, Cameron RE. Chitosan/ apatite composite beads prepared by in situ generation of apatite or Siapatite nanocrystals. Acta Biomaterialia 2010;6:466-76.
19. Guadarrama D. Preparación y caracterización de cementos óseos acrílicos modificados con micro y nanopartículas de hidroxiapatita. Tesis de Diploma. Universidad de La Habana, La Habana, Cuba, 2009.
20. Xie JZ, Hein S, Wang K, Liao K, Goh KL. Influence of hydroxyapatite crystallization temperature and concentration on stress transfer in wet-spun nanohydroxyapatitechitosan composite fibres. Biomed Mater 2008;3(2):25014.
21. Li J, ChenY, Yin Y, Yao F, Yao K. Modulation of nano-hydroxyapatite size via formation on chitosan-gelatin network film in situ. Biomaterials 2007;28(5):781-90.
22. Kawachi E, Betran CA, Dos Reis RR, Alves OL. Biocerâmicas: tendências e perspectivas de uma área interdisciplinar. Quim Nova 2000;23:518-22.
23. Alonso LM. Preparación de monolitos de fosfato de calcio por vía hidrotermal. Tesis en opción al grado de Máster en Ciencia y Tecnología de Materiales. Universidad de La Habana, La Habana, Cuba, 2005.
24. Delgado JA, Harr I, Almirall A, del Valle S, Planell JA, Ginebra MP. Injectability of a macroporous calcium phosphate cement. Key Eng Mater 2005; 284-6:157-60.
25. Bauer T, Togawa D. Bone graft substitutes: Towards a more perfect union. Orthopedics 2003;26:925-6.
26. Motz W, Bentley C, Tasto J. Bioabsorbable implants in orthopaedics: New developments and clinical circone applications. J Am Acad Orthop Surg 2001;9:280-8.
27. Pastor de Abram A, Higuera I. Generalidades in quitina y quitosano: obtención, caracterización y aplicaciones. Pastor A, editor. Pontificia Universidad Católica de Perú, Lima; 2004, p. 22.
28. Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005;26:5983-90.
29. Prasitsilp M, Jenwithisuk R, Kongsuwan K, Damrongchai N, Watts P. Cellular responses to chitosan in vitro: the importance of deacetylation. J Mater Sci Mater Med 2000;11:773-8.
30. Vande VPJ, Matthew HWT, Desilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of chitosan scaffold. J Biomed Mater Res 2002;59:585-90.
31. Dimitriu S. Polymeric Biomaterials. Technology and Engineering, Marcel Dekker, Inc. New York; 2002, p. 37.
32. Tangsadthakun C, Kanokpanont S, Sanchavanakit N, Pichyangkura R, Banaprasert T, Tabata Y, et al. The influence of molecular weight of chitosan on the physical and biological properties of collagen/chitosan scaffolds. J Biomater Sci Polymer Ed 2007;18(2):147-63.
33. Sechriest VF, Miao YJ, Niyibizi C, Westerhausen-Larson A, Matthew HW, Evans CH, et al. GAG-augmented polysaccharide hydrogel: a novel compatible and biodegradable material to support chondrogenesis. J Biomed Mater Res 2000;49(4):534-41.
34. Cui YL, Qi AD, Liu WG, Wang XH, H. Wang, DM Ma, et al. Biomimetic surface modification of poly(L-latic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials 2003;24:3859-68.
35. Lee JE, Kim KE, Kwon IC, Ahn HJ, Lee SH, Cho H, et al. Effects of the controlledreleased TGF-beta 1 from chitosan microspheres on chondrocytes cultured in collagen/ chitosan/glycosaminoglycan scaffold. Biomaterials 2004;25:4163-73.
36. Aimin C, Chunlin H, Juliang B, Tinyin Z, Zhichao D. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin Orthop 1999;366:239-47.
37. Hu SG, Jou CH, Yang MC. Protein adsorption, fibroblast activity and antibacterial properties of poly(3-hydroxybutric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immovilized hyaluronic acid. Biomaterials 2003;24:2685-93.
38. Sano H, Shibasaki K, Matsukubo T, Takaesu Y. Effect of molecular mass and degree of deacetylation of chitosan on adsorption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. Bull Tokio Dent Coll 2002;43(2):75-82.
39. Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan. Effect of pH, serum, and molecular mass of chitosan on transfection efficience. Biomaterials 2001;22:2075-80.
40. Tachaboonyakiat W, Ogomi D, Serizama T, Akashi M. Evaluation of cell adhesion and proliferation on a novel tissue engineering scaffold containg chitosan and hydroxyapatite. J Bioact Compat Pol 2006;21:579-89.
41. Mao JS, Cui YL, Wang XH, Sun Y, Yin YJ, Zhao HM, et al. A preliminary study on chitosan and gelatin polyelectrolite complex cytocompatibility by cell cycle and apoptosis analysis. Biomaterials 2004; 25:3973-81.
42. Griffon DJ, Sedighi MR, Schaeffer DV, Eurell JA, Johnson AL. Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater 2006; 2:313-20.
43. Kosaraju S, D´ath L, Lawrence A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr Polym 2006;64:163-7.
44. Vande V, Matthew HWT, Desilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of chitosan scaffold. J Biomed Mater Res 2002;59:585-90.
45. Hyeong-Ho J, Chang-Hun L, Won-Ki L, Jin-Kook L, Hong-Chae P, Seog-Young Y. In situ formation of the hydroxyapatite/ chitosan-alginate composite scaffolds. Mater Lett 2008;62(10-11):1630-3.
46. Sun L, Xu HH, Takagi S, Chow LC. Fast setting calcium phosphate cementchitosan composite: mechanical properties and dissolution rates. J Biomater Appl 2007;21(3):299-315.
47. Chesnutt B, Viano AM, Yuan Y, Yang Y, Guda T, Appleford MR, et al. Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration. J Biomed Mater Res A 2009;88(2):491-502.
48. Hua Y, Ning C, Xiaoying L, Buzhong Z, Wei C, Xiaoling S. Natural hydroxyapatite/ chitosan composite for bone substitute materials. Eng Med Biol Soc 2005; 5:4888-91.
49. Nie H, Wang CH. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J Control Release 2007;120(1-2): 111-21.
50. Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release 2009;134(2):111-7.
51. Murugan R, Ramakrishna S. Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials 2004;25:3829-35.
52. Finisie M, Josué A, Fávere VT, Laranjeira MCM. Synthesis of calcium-phosphate and chitosan bioceramics for bone regeneration. An Acad Bras Cienc 2001; 73(4):525-32.
53. Granja P, Silva AIN, Borges JP, Barrias CC, Amaral IF. Preparation and characterization of injectable chitosan-hydroxyapatite microspheres. Key Eng Mater 2004; 254-6:573-6.
54. Carey L, Xu HHK, Simon CG, Takagi S, Chow LC. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials 2005;26:5002-14.
55. Xu H, Simon CG Jr. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials 2005;26:1337-48.
56. Lian Q, Li DC, He JK, Wang Z. Mechanical properties and in vivo performance of calcium phosphate cement-chitosan fibre composite. Proc Inst Mech Eng (H) 2008; 222(3):347-53.
57. Zou Q, Li Y, Zhang L, Zuo Y, Li J, Li X. Characterization and cytocompatibility of nano-hydroxyapatite/chitosan bone cement with the addition of calcium salts. J Biomed Mater Res B Appl Biomater 2009; 90(1):156-64.
58. Padois K, Rodriguez F. Effects of chitosan addition to self-setting bone cement. Biomed Mater Eng 2007;17(5):309-20.
59. Teng S, Lee EJ, Wang P, Shin DS, Kim HE. Three-layered membranes of collagen/ hydroxyapatite and chitosan for guided bone regeneration. J Biomed Mater Res B Appl Biomater 2008;87(1):132-8.
60. Weir M, Xu HH. High-strength in situsetting calcium phosphate composite with protein release. J Biomed Mater Res A 2008;85(2):388-96.
61. Xu H, Quinn JB, Takagi S, Chow LC. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials 2004;25(6):1029-37.
62. Zhang Y, Xu HH. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J Biomed Mater Res A 2005;75(4):832-40.
63. Xu H, Takagi S, Sun L, Hussain L, Chow LC, Guthrie WF, et al. Development of nonrigid, durable calcium phosphate cement for use in periodontal bone repair. J Am Dent Assoc 2006;137(8):1131-8.
64. Fuentes G, Rojas ML, Delgado JA, Llópiz JC, Peón E. Physical chemical and thermoanalytical characterization of cements based on synthetic hydroxyapatite. Rev Cenic Cienc Quím 2006;37(2):63-8.
65. Fuentes G, Hernández Y, Campos Y, López N, Rojas NM, Peón E, et al. Composition influence on properties of acrylic composites loaded with synthetic hydroxyapatite. Lat Am Appl Res 2008;38:105-12.
66. Pang X, Zhitomirsky I. Electrophoretic deposition of composite hydroxyapatite- chitosan coatings. Mater Character 2007;58(4):339-48.
67. Sun F, Sask KN, Brash JL, Zhitomirsky I. Surface modifications of Nitinol for biomedical applications. Colloids Surf B Biointerfaces 2008;67(1):132-9.
68. Redepenning J. Electrolytic deposition of coatings for prosthetic metals and alloys. US Patent 7387846. June 17th, 2008.
69. Vogt J, Brandes G, Krüger I, Behrens P, Nolte I, Lenarz T, et al. Comparison of different nanostructured biomaterials in subcutaneous tissue. J Mater Sci Mater Med 2008;19(7):2629-36.
70. Turck C, Brandes G, Krueger I, Behrens P, Mojallal H, Lenarz T, et al. Histological evaluation of novel ossicular chain replacement prostheses: an animal study in rabbits. Acta Otolaryngol 2007;127(8):801-8.
71. Manjubala I, Woesz A, Pilz C, Rumpler M, Fratzl-Zelman N, Roschger P, et al. Biomimetic mineral-organic composite scaffolds with controlled internal architecture. J Mater Sci Mater Med 2005;16(12):1111-9.
72. Kong L, Ao Q, Wang A, Gong K, Wang X, Lu G, et al. Preparation and characterization of a multilayer biomimetic scaffold for bone tissue engineering. J Biomater Appl 2007;22(3):223-39.
73. Teng S, Lee EJ, Wang P, Jun SH, Han CM, Kim HE. Functionally gradient chitosan/hydroxyapatite composite scaffolds for controlled drug release. J Biomed Mater Res B Appl Biomater 2009;90(1):275-82.
74. Tang Y, Du Y, Li Y, Wang X, Hu X. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing hydroxyapatite for protein delivery.
J Biomed Mater Res A 2009;91(4):953-63.
75. Yamaguchi I., Tokuchi K, Fukusaki H, Koyama Y, Takakura K, Monma H, et al. Preparation and microstructure analysis of chitosan/hydroxiapatite nanocomposites. J Biomed Mater Res 2001;55:20-7.
76. Fan Y, Lü X. A study of apatite formation on natural nano-hydroxyapatite/chitosan composite in simulated body fluid. Front Mater Sci China 2008;2(1):91-4.
77. Redepenning J, Venkataraman G, Chen J, Stafford N. Electrochemical preparation of chitosan/hydroxyapatite composite coatings on titanium substrates. J Biomed Mat Res 2003;66A:411-6.
78. Hu Q, Li B, Wang M, Shen J. Preparation and characterization of biodegradable chitosan/ hydroxyapatite nanocomposite rods via in situ hybridization: a potential material as internal fixation of bone fracture. Biomaterials 2004;25:779-85.
79. Rusu V, Wilke Ch, Ng M, Tiersch B, Fratzl P, Peter MG. Size-controled hydroxyapatite nanoparticles as self-organized organicinorganic composite materials. Biomaterials 2005; 26:5414-26.
80. Chang M, Ko CC, Douglas WH. Preparation of hydroxyapatite-gelatine nanocomposites. Biomaterials 2003;24(17):2853-62.
81. Peña J, Izquierdo-Barba I, Martínez A, Vallet- Regí M. New method to obtain chitosan/apatite materials at room temperature. Solid State Sci 2006;8:513-9.
82. Van der Houwen J, Cressey G, Cressey BA, Valsami-Jones E. The effect of organic ligands on the crystallinity of calcium phosphate. J Cryst Growth 2003;249:572-83.
83. Solís Y, García R, Davidenko N, Peniche C, Camerón R. Preparación y caracterización de composites de hidroxiapatita deficiente en calcio y quitosana. Rev Cenic Cienc Quím 2008;39(1):17-22.
84. Davidenko N, Carrodeguas RG, Peniche C, Solís Y, Cameron RE. Chitosan/apatite composite beads prepared by in situ generation of apatite or Si-apatite nanocrystals. Acta Biomaterialia 2010;6:466-76.
85. Murugan R, Kumar TSS, Yang F, Ramakrishna S. Hydroxyl carbonate apatite hybrid bone composites using carbohydrate polymer. J Compos Mater 2005;39(13):1159-67.
86. Katti K, Katti DR, Dash R. Synthesis and characterization of a novel chitosan/montmorillonite/ hydroxyapatite nanocomposite for bone tissue engineering. Biomed Mater 2008;3(3):034122.
87. Mano J, Sousa RA, Boesel LF, Neves NM, Reis RL. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos Sci Technol 2004;64:789-817.
88. Freier T, Shan KH, Kazazian K, Shoichet M. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005;26:5872-8.
89. Muramatsu K, Masuda S, Yoshihara Y, Fujisawa A. In vitro degradation behavior of freeze-dried carboxymethyl-chitin sponges processed by vacuum-heating and gamma irradiation. Polym Degrad Stabil 2003;81: 327-32.
90. Tachaboonyakiat W, Serizawa T, Akashi M. Inorganic-organic polymer hybrid scaffold for tissue engineering-II: partial enzymatic degradation of hydroxyapatite-chitosan hybrid. J Biomater Sci Polym Ed 2002;13(9):1021-32.
91. Zhou G, Li Y, Zhang L, Zuo Y, Jansen JA. Preparation and characterization of nanohydroxyapatite/ chitosan/konjac glucomannan composite. J Biomed Mater Res A 2007; 83(4):931-9.
92. Weir M, Xu HH, Simon CG Jr. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J Biomed Mater Res A 2006;77(3): 487-96.
93. Yang Y, Hu W, Wang XD, Gu XS. The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo. J Mater Sci Mater Med 2007;18:2117-21.
94. Chatelet C, Damour O, Domard A. Infuence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001;22:261-8.
95. Liuyun J, Yubao L, Li Z, Jianguo L. Preparation and properties of a novel bone repair composite: nano-hydroxyapatite/chitosan/ carboxymethyl cellulose. J Mater Sci Mater Med 2008;19(3):981-7.
96. Araújo A, Lemos AF, Ferreira JM. Rheological, microstructural, and in vitro characterization of hybrid chitosan-polylactic acid/ hydroxyapatite composites. J Biomed Mater Res A 2009;88(4):916-22.
97. Teng S, Lee EJ, Yoon BH, Shin DS, Kim HE, Oh JS. Chitosan/nanohydroxyapatite composite membranes via dynamic filtration for guided bone regeneration. J Biomed Mater Res A 2009;88(3):569-80.
98. Yang D, Jin Y, Zhou Y, Ma G, Chen X, Lu F, et al. In situ mineralization of hydroxyapatite on electrospun chitosan-based nanofibrous scaffolds. Macromol Biosci 2008;8(3):239-46.
99. Sivakumar M, Rao KP. Preparation, characterization, and in vitro release of gentamicin from coralline hydroxyapatite-alginate composite microspheres. J Biomed Mater Res A 2003;65(2):222-8.
100. Krisanapiboon A, Buranapanitkit B, Oungbho K. Biocompatibility of hydroxyapatite composite as a local drug delivery system. J Orthop Surg 2006;14(3):315-8.
101. Tang X, Gui L, Lü XY. Hard tissue compatibility of natural hydroxyapatite/chitosan composite. Biomed Mater 2008;3(4):44115.
102. Vechasilp J, Tangtrakulwanich B, Oungbho K, Yuenyongsawad S. The efficacy of methotrexate-impregnated hydroxyapatite composites on human mammary carcinoma cells. J Orthop Surg 2007;15(1):56-61.
103. Inanç B, Eser-Elçin A, Koç A, Baloş K, Parlar A, Murat-Elçin Y. Encapsulation and osteoinduction of human periodontal ligament fibroblasts in chitosan-hydroxyapatite microspheres. J Biomed Mater Res A 2007; 82(4):917-26.
104. Sailaja G, Ramesh P, Kumary TV, Varma HK. Human osteosarcoma cell adhesion behaviour on hydroxyapatite integrated chitosanpoly( acrylic acid) polyelectrolyte complex. Acta Biomater 2006;2(6):651-7.
105. Wu J, Liu Y, Yang TF, Mu YH, Guo T, Li YB. Porous polyvinyl alcohol hydrogel composite prepared and studied initially for biocompatibility. Sichuan Da Xue Xue Bao Yi Xue Ban 2007;38(4):705-8.
106. Kong L, Gao Y, Cao W, Gong Y, Zhao N, Zhang X. Preparation and characterization of nano-hydroxyapatite/chitosan composite scaffolds. J Biomed Mater Res A 2005; 75(2):275-82.
107. Li Z, Yubao L, Aiping Y, Xuelin P, Xuejiang W, Xiang Z. Preparation and in vitro investigation of chitosan/nano-hydroxyapatite composite used as bone substitute materials. J Mater Sci Mater Med 2005;16(3):213-9.
108. Fulmer M, Ison I, Hankermayer C, Constantz B, Ross J. Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials 2002;23:751-5.
109. Gallardo A, Aguilar MR, Elvira C, Peniche C, San Román J. Chitosan based microcomposites from biodegradable microparticles to selfcuring hydrogels. In: Biodegradable Systems in Tissue Engineering, CRC Press: Boca Ratón, USA, SRJ Reis R, editor. 2005, p. 145-62.
110. Buranapanitkit B, Srinilta V, Ingviga N, Oungbho K, Geater A, Ovatlarnporn C. The efficacy of a hydroxyapatite composite as a biodegradable antibiotic delivery system. Clin Orthop Relat Res 2004;424:244-52.
111. Baran E, Tuzlakoglu K, Salgado AJ, Reis RL. Multichannel mould processing of 3D structures from microporous coralline hydroxyapatite granules and chitosan support materials for guided tissue regeneration/engineering. J Mater Sci Mater Med 2004;15(2):161-5.
112. Zhao F, Grayson WL, Ma T, Bunnell B, Lu WW. Effects of hydroxyapatite in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006;27(9):1859-67.
113. Zhang Y, Zhang M. Cell growth and function on calcium phosphate reinforced chitosan scaffolds. J Mater Sci Mater Med 2004;15(3):255-60.
114. Zhang Z, Ni M, Zhang M, Ratner B. Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng 2003;9(2):337-45
115. Zhao F, Yin Y, Lu WW, Leong JC, Zhang W, Zhang J, et al. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials 2002; 23(15):3227-34.
116. Zhao F, Yin YJ, Yao KD, Guo G, Wang BL, Zhang JY, et al. Tissue engineering study on chitosan-gelatin/hydroxyapatite composite scaffolds-osteoblasts culture. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2002;16(2):130-3.
117. Zhao F, Yin YJ, Song XF. Study on chitosan-gelatin/hydroxyapatite composite scaffolds preparation and morphology. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2001; 15(5):276-9.
118. Maachou H, Bal KE, Bal Y, Chagnes A, Cote G, Alliouche D. Characterization and in vitro bioactivity of chitosan/hydroxyapatite composite membrane prepared by freezegelation method. Trends Biomater Artif Organs 2008; 22(1):15-24.
119. Kim S, Lim B, Sun F, Koh K, Ryu S, Kim H, et al. Preparation of high flexible composite film of hydroxyapatite and chitosan. Polym Bull 2009;62(1):111-8.
120. Jiang L, Li Y, Wang X, Zhang L, Wen J, Gong M. Preparation and properties of nano-hydroxyapatite/chitosan/carboxymethyl cellulose composite scaffold. Carbohydr Polym 2008;74(3):680-4.
121. Xiao X, Liu R, Huang Q. Preparation and characterization of nano-hydroxyapatite/polymer composite scaffolds. J Mater Sci Mater Med 2008;19(11):3429-35.
122. Zhang Y, Xu HHK, Takagi S, Chow LC. In situ hardening hydroxyapatite-based scaffold for bone repair. J Mater Sci Mater Med 2006; 17(5):437-45.
123. Xu HH, Quinn JB, Takagi S, Chow LC. Processing and properties of strong and nonrigid calcium phosphate cement. J Dent Res 2002;81(3):219-24.
124. Wang J, De Boer J, De Groot K. Proliferation and differentiation of MC3T3-E1 cells on calcium phosphate/chitosan coatings. J Dent Res 2008;87(7):650-4.
125. Huang Z, Dong Y, Chu C, Lin P. Electrochemistry assisted reacting deposition of hydroxyapatite in porous chitosan scaffolds. Mater Lett 2008;62(19):3376-8.
126. Kumar R, Prakash KH, Cheang P, Gower L, Khor KA. Chitosan-mediated crystallization and assembly of hydroxyapatite nanoparticles into hybrid nanostructured films. J R Soc Interface 2008;5(21):427-39.
127. Chen F, Wang Z, Lin C. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nanocomposite for use in biomedical materials. Mater Lett 2002;57(4):858-61.
128. Kong L, Gao Y, Lu G, Gong Y, Zhao N, Zhang X. A study on the bioactivity of chitosan/ nano-hydroxyapatite composite scaffolds for bone tissue engineering. Eur Polym J 2006; 42(12):3171-9.
129. Pramanik N, Mishra D, Banerjee I, Maiti TK, Bhargava P, Pramanik P. Chemical synthesis, characterization, and biocompatibility study of hydroxyapatite/chitosan phosphate nanocomposite for bone tissue engineering applications. Int J Biomater 2009; 2009:512417.
Received in November, 2009.
Accepted for publication in July, 2010.




![Erratum: Experiencias del Programa de atención integral a pacientes con pie diabético en el estado Zulia, Venezuela [Biotecnol Apl;27(2):101-9]](/img/es/prev.gif)