Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Biotecnología Aplicada
versión On-line ISSN 1027-2852
Biotecnol Apl vol.30 no.2 La Habana abr.-jun. 2013
RESEARCH
Immobilization of pectinase by adsorption on an alginate-coated chitin support
Inmovilización de pectinasa por absorción en matriz de quitina recubierta con alginato
Hector L Ramirez1, Ana I Briones2, Juan Úbeda2, María Arevalo2
1 Centro de Tecnología Enzimática, Universidad de Matanzas Camilo Cienfuegos, Autopista a Varadero, Km 3½, CP 44740, Matanzas, Cuba.
2 Departamento de Química Analítica y Tecnología de los Alimentos, Universidad de Castilla-La Mancha. Ave. Camilo José Cela, 10, 13071, Ciudad Real, España.
ABSTRACT
Aspergillus niger pectinase was immobilized on an alginate-coated chitin support by adsorption. The optimal conditions for immobilization were: pH 4.5, time of incubation 2 h and 85 µg/mL protein concentration. The yield of immobilized protein was 70 % and the enzyme retained 60 % of the initial activity. Optimal pH, heat stability and reusability were evaluated, among other properties of the immobilized enzyme. The thermostability was enhanced by about 9.7 °C after immobilization. The immobilized pectinase was resistant during incubation, 10-fold more resistant to thermal treatment at 50 ºC than the native enzyme. The optimal pH for the catalytic activity of both, the immobilized pectinase and the free enzyme, was the same, the prepared biocatalyst retaining 50 % of the original catalytic activity after 9 cycles of reuse. The obtained bioconjugate showed good operational stability and improved thermostability. These properties support the potential application of the immobilized pectinase at the juices industry.
Keywords: pectinase, adsorption, chitin, alginate, enzyme stability, enzyme immobilization.
RESUMEN
Se inmovilizó la pectinasa de Aspergillus niger, por absorción en un soporte de quitina recubierta con alginato. Las condiciones óptimas fueron pH 4.5, incubación 2 h y concentración de proteína 85 µg/mL. El rendimiento de la proteína inmovilizada fue del 70 % y la enzima retuvo el 60 % de su actividad inicial. Se evaluaron el pH óptimo, la termoestabilidad y la reusabilidad, entre otras propiedades de la enzima inmovilizada. La termoestabilidad cambió en 9.7 °C, y la enzima inmovilizada fue 10 veces más estable ante el tratamiento térmico a 50 °C que la enzima nativa. El pH óptimo para la actividad catalítica de la pectinasa inmovilizada y la libre fue el mismo. El biocatalizador retuvo el 50 % de su actividad catalítica original después de 9 ciclos de reuso. El bioconjugado mostró estabilidad operacional y mejoramiento de la termoestabilidad. Estas propiedades son adecuadas para la potencial aplicación de la enzima inmovilizada en la industria de jugos.
Palabras clave: pectinasa, absorción, quitina, alginato, estabilidad de enzimas, inmovilización de enzimas.
INTRODUCTION
The important properties of enzymes that make them of great interest as industrial catalysts are specificity and high activity. The use of enzymes in this field requires their easy recovery from the reaction mixture at the end of the procedure, the catalytic activity remaining stable over long periods of time and applicability to continuous processes. All these requirements may be guaranteed by enzyme immobilization.
Immobilized enzymes find broad application in industry, biotechnology, biomedicine and analytical chemistry [1-4]. Generally, they show better thermal and pH stabilities and are easier to separate, can be reused and their effect appears to be more suitable for practical applications [5]. Various techniques have been developed for enzyme immobilization, including adsorption to insoluble materials, entrapment in polymeric gels, encapsulation in membranes, crosslinking with bifunctional reagent, or covalent linking to an insoluble carrier [6-10].
Enzymes cleaving the pectic substances are broadly known as pectinolytic enzymes or pectinases [11]. They are classified on the basis of the mechanism used to “attack” the galacturonan backbone as polysaccharide hydrolases, polysaccharide lyases, and carbohydrate esterases. These enzymes include endopolygalacturonases (EC 3.2.1.15), exopolygalacturonases (EC 3.2.1.67), pectate lyases (EC 4.2.2.2), pectin lyases (EC 4.2.2.10) and pectin methyl esterases (EC 3.1.1.11)[12]. Polygalacturonase enzymes belong to the group of depolymerising hydrolytic pectinases, and they are able to hydrolyse pectin and/or pectic acid. These enzymes have widespread applications in the food industry (processing of fruits), wastewater treatment, textile industries, fruit softening and plant infection processes [13-16].
The stability of these enzymes depends on the aqueous medium, and is easily disrupted to the point where the enzymes cannot function appropriately. Immobilization techniques provide a promising approach to retain their stability. Various methods for immobilization of this enzyme have been described: entrapping in alginate [17], physical adsorption on anion resin [18], γ-alumina [19], particles and nanoparticles of silica [20, 21] and covalent attachment to carriers such as porous glass [22] and nylon [23]. However, the development of new methods and supports for immobilizing enzymes receives special importance in enzyme technology.
In the present paper we describe a method for immobilization of pectinase by adsorption on an alginate-coated chitin support, previously synthesized in our laboratory [24]. To our knowledge, the immobilization of pectinase on this type of support has not been reported.
MATERIALS AND METHODS
Materials
Sodium alginate from Laminaria hyperborea was purchased from BDH (Poole, UK). Analytical data were: molecular weight of 1.97 × 105 Da [25], uronate composition of 37.5 % mannuronate, 62.5 % guluronate [26]. Commercially available soluble pectinase (E.C.3.2. 1.15; 316 U/mg) from Aspergillus niger was purchased from Agrovin. S.A. (Ciudad Real, Spain). Chitin from lobster shells (degree of deacetylation of 10 % [27], average particle size of 30 µm) was obtained by the Mario Muñoz Pharmaceutical Laboratories (Havana, Cuba). The 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) was obtained from Merck. All other chemicals used were of analytical grade.
Enzyme immobilization
Alginate-coated chitin support was prepared as previously described [24]. Briefly, 600 mg of the anionic polysaccharide were dissolved in 60 mL of potassium phosphate buffer, pH 6.0 and then 150 mg EDAC were added. The solution was stirred for 1 h at room temperature and further mixed with a suspension of chitin (3 g) in 30 mL distilled water. The reaction was maintained at 25 ºC for 16 h under continuous stirring. The solid was collected by centrifugation, washed several times with distilled water until carbohydrates were not detected in the wastes, and finally suspended in 90 mL of 50 mM sodium acetate buffer, pH 4.5.
Thirty milligrams of pectinase were added to the solution containing the alginate-coated support and the mixture was stirred during 2 h at 4 ºC. After the immobilization, the support was washed repeatedly with 20 mM sodium acetate buffer, pH 4.5 and the amount of adsorbed enzyme was estimated by difference after measuring the non-immobilized protein.
Assays
The enzymatic activity of native and modified pectinase was determined by adding 100 μL of enzyme solution to 400 μL of 0.5 % (w/v) polygalacturonic acids in 20 mM sodium acetate buffer, pH 4.2. For immobilized samples, 100 μL of a suspension containing the same amount of enzyme protein was used. After 30 min at 37 ºC the reaction was stopped by adding 3,5- dinitrosalicylic acid and the reducing sugars were determined colorimetrically after boiling the solutions during 10 min [28]. One unit of activity was defined as the amount of enzyme required to hydrolyze 1.0 μmol polygalacturonic acids per min under the described conditions. Pectinase concentration was estimated by the Bradford method [29].
Determination of kinetic parameters
To determine the Michaelis–Menten constant (Km), the activity assay was determined in different substrate (polygalacturonic acids) concentrations. Polygalacturonic acids solutions (0.025-0.25 %, w/v) were prepared in acetate buffer 0.02 mol/L, pH 4.2, and kept in a water bath at 37 ºC for 30 min. Subsequently, the immobilized pectinase or free enzyme solution was added to the test tubes and shaken for different incubation times.
Optimum pH
The hydrolytic activity of the enzyme preparations (0.5 U/mL final concentration, corresponding to 100 % in the graphic) on polygalacturonic acids were measured at 37 ºC in 50 mM acetic acid/sodium acetate buffer solution at pH ranging from 3 to 6.
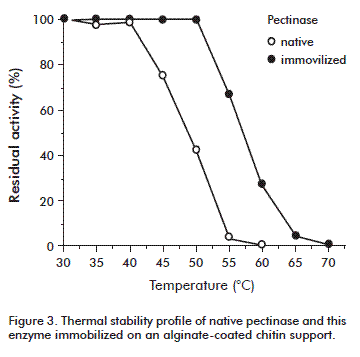
Thermal stability profile
Pectinase forms were incubated at selected temperatures from 35 to 70 ºC in 20 mM sodium acetate buffer, pH 4.5 (0.5 U/mL final concentrations, corresponding to 100 % in the graphic). Aliquots were removed after 10 min incubation, quickly chilled, and assayed for enzymatic activity.
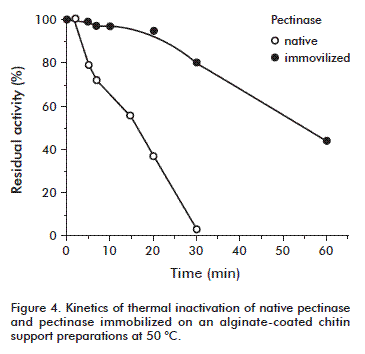
Kinetics of thermal inactivation at 50 ºC
The enzymes were incubated at 50 ºC in 20 mM sodium acetate buffer, pH 4.5 (0.5 U/mL final concentration corresponding to 100 % in the graphic). Aliquots were removed at scheduled times, quickly chilled, and assayed for enzymatic activity. The half-life times were calculated from the first-order rate constants of inactivation, ki, obtained from linear regression in logarithmic coordinates.
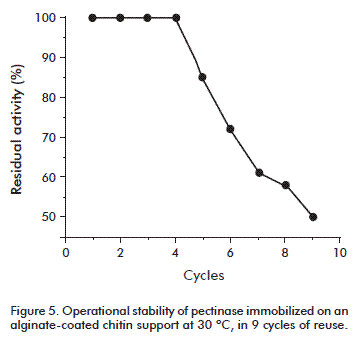
Reuse of immobilized pectinase
The reuse of immobilized pectinase was assessed by carrying out the hydrolysis of 0.25 % (w/v) apple pectin (prepared with pH 4.5 acetate buffer, 20 mM) at 30 ºC while stirring at 120 rpm; this procedure was performed in consecutive cycles while repeatedly reusing the enzyme. After each 30 min. cycle, the immobilized pectinase was washed with acetate buffer (pH 4.5). The activity of the immobilised enzyme was expressed as a percentage of its residual activity compared to the initial activity in the first cycle.
Statistical analysis
All the results showed were performed at last three times, reporting average values for these determinations. Hypothesis tests were performed to compare average data using the Student´s t distribution with a 95 % confidence interval (when significant differences in the average values were obtained). Mean values and standard deviations were calculated and represented with the aid of the Origin 7.0 program (NCSS, USA).
RESULTS AND DISCUSSION
The effects of the following parameters on the immobilization process were investigated: solution pH, time of pectinase immobilization and protein concentration. Figure 1A shows the effect of solution pH on enzyme activity during the immobilization process. In this case, the higher catalytic activity for the immobilized pectinase was observed at pH 4.5, this value being set for further experiments. Figure 1B shows the time course of pectinase immobilization. The immobilization degree of the enzyme was high even at low incubation times, but the maximum immobilized activity was observed after 2-h incubation. The effect of the bulk solution initial protein concentration on the immobilized amount of pectinase was determined by incubating the enzyme with the support for 2 h at 4 ºC, pH 4.5. As shown in figure 1C, the immobilized activity increases progressively with the increase in pectinase concentration, reaching maximal activity at protein concentrations higher than 85 μg/mL. Consequently, this value of initial pectinase concentration was selected as optimum for further experiments. Under these conditions, the yield of immobilized protein was determined as 7.9 mg/g support, representing 70 % of the initial amount of incubated enzyme. The immobilized pectinase retained 60 % of the initial specific activity of the native enzyme.
The apparent Km of the immobilized enzyme was 0.98 g/L, and 0.94 g/L for the free enzyme. This suggests that the affinity of the active site of the immobilized enzyme for the substrate was the same as that of the free enzyme, probably because of no substantial changes on the enzyme conformation during immobilization. Similar results were obtained for immobilization of pectin lyase from Aspergillus niger in alginate beads [30] and glutaraldehyde-activated bentonite [31].
The pH dependence of the activity of native and immobilized pectinase was studied at 30 ºC in the pH range 2-6. As shown in figure 2, the pH optimum for the catalytic activity of both immobilized pectinase and the free enzyme was the same, in the range 4.5-5.0. These results suggest that the immobilization process did not affect the pH optimum for catalytic activity of the enzyme significantly.
Thermal stability experiments were carried out with free and immobilized enzyme, by incubating their samples in the absence of substrate at several temperatures. The thermotolerance of native and immobilized pectinase was evaluated from the activity retained after heating the enzymes at different temperatures for 10 min. Under these conditions, the thermostability of immobilized pectinase was improved, as shown in figure 3. The native enzyme was inactive at 60 °C, while the immobilized enzyme retained over 25 % of its initial activity at that temperature. A complete loss of activity for modified pectinase was observed by incubating it at 70 °C. The calculated value for T50, defined as the temperature at which 50 % of the initial activity was retained, was increased from 48.3 to 58 ºC for the enzyme after immobilization on the negatively-charged support.
The heat stability of both enzyme variants at 50 °C is shown in Figure 4. All pectinase preparations lost activity progressively according to a monophasic inactivation kinetics. The immobilized pectinase showed a reduced enzymatic activity over time, retaining more than 44 % of its initial activity after 60 min of incubation at 50 °C. However, the enzymatic activity of the native pectinase decreased and was completely lost after 40 min of incubation at this temperature. The half-life time of pectinase increased from 13.5 to 138 h after immobilization on the solid support. This improved thermal stability of the prepared conjugate could be explained by the maintenance of the active enzyme conformation, since the support material was supposed to preserve the tertiary structure of the enzyme. This is also in agreement with the results shown above. Moreover, it could be pointed out that the thermal stability of an enzyme may indicate the efficiency of the immobilization method and also reflect the delicate balance between the acquired conformational stability and the resulting microenvironment surrounding the enzyme.
The reuse of this biocatalyst was studied, since this factor is essential for the applications of the enzyme. The reusability of the immobilized preparation was assessed by carrying out the hydrolysis of 0.25 % pectin (at 30 °C). In this case, the immobilized enzyme showed stable when it was repeatedly used for pectin hydrolysis, retaining near 50 % of its initial activity after 9 cycles of reuse (Figure 5). Therefore, the retention of the catalytic activity of the immobilized enzyme derived from its adsorption to the alginate-coated support.
CONCLUSIONS
In the present work, we described a new bioconjugate obtained by the non-covalent immobilization of pecti-nase on an alginate-coated chitin support. The pectinase enzyme preparation is commercially available and is fairly inexpensive. The biocatalyst prepared by loading pectinase on sodium alginate-coated chitin showed increased thermostability compared to the native enzyme. The optimum pH range for catalytic activity was the same for both variants, the free and immobilized pectinase. This biocatalyst showed a good operational stability. The results presented here suggest that enzyme adsorption on alginate-coated chitin support may be useful to improve the functional and operational properties of other enzymes.
ACKNOWLEDGMENTS
HL Ramirez thanks the Castilla La Mancha University for supporting his stage in Ciudad Real (2007, 2008 and 2010) and the fellowship from AECI, Spain (2009). The authors acknowledge the financial support from the Castilla La Mancha government (bilateral project POII10-0085-5338) and the International Foundation for Science, Stockholm, Sweden (Grant F/3004-67). We are also grateful to Dr. Reynaldo Vi-llalonga Santana for his valuable technical assistance.
REFERENCES
1. Yagiz F, Kazan D, Nilgun A. Biodiesel production from waste oils by using lipase immobilized on hydrotalcite and zeolites. Chem Eng J. 2007;134(1):262-7.
2. Erhardt FA, Jordening HJ. Immobilization of dextranase from Chaetomium erraticum. J Biotechnol. 2007;131(4):440-7.
3. Wen Z, Niemeyer B. Evaluation of two different Concanavalin A affinity adsorbents for the adsorption of glucose oxidase. J Chromatogr B Anal Technol Biomed Life Sci. 2007;857(1):149-57.
4. Camacho C, Matias JC, Chico B, Cao R, Gomez L, Simpson BK, et al. Amperometric biosensor for Hydrogen Peroxide, using supramolecularly immobilized Horseradish Peroxidase on the β-Cyclodextrin-coated gold electrode. Electroanalysis. 2007;19(24):2538-42.
5. Ye P, Jiang J, Xu ZK. Adsorption and activity of lipase from Candida rugosa on the chitosan-modified poly(acrylonitrile-co-maleic acid) membrane surface. Colloids Surf B Biointerfaces. 2007;60(1):62-7.
6. Gomez L, Ramírez HL, Cabrera G, Simpson B, Villalonga R. Immobilization of invertase-chitosan conjugate on hyaluronic acid-modified chitin. J Food Biochem. 2008;32(2):264-77.
7. Vaidya B, Karale A, Suthar H, Ingavle G, Pathak T, Ponrathnam S, et al. Immobilization of mushroom polyphenol oxidase on poly(allyl glycidyl ether-co-ethylene glycol dimethacrylate) macroporous beaded copolymers. React Funct Polym. 2007; 67(10):905-15.
8. Huang XJ, Ge D, Xu ZK. Preparation and characterization of stable chitosan nanofibrous membrane for lipase immobilization. Eur Polym J. 2007;43(9):3710-8.
9. Haider T, Husain Q. Calcium alginate entrapped preparations of Aspergillus oryzae beta galactosidase: its stability and applications in the hydrolysis of lactose. Int J Biol Macromol. 2007;41(1):72-80.
10. Lopez-Gallego F, Betancor L, Mateo C, Hidalgo A, Alonso-Morales N, Dellamora-Ortiz G, et al. Enzyme stabilization by glutaraldehyde crosslinking of adsorbed proteins on aminated supports. J Biotechnol. 2005;119(1):70-5.
11. Saad N, Briand M, Gardarin C, Briand Y, Michaud P. Production, purification and characterization of an endopolygalacturonase from Mucor rouxii NRRL 1894. Enzyme Microb Technol. 2007;41(6-7):800-5.
12. Alkorta I, Garbisu G, Llama MJ, Serra JL. Industrial applications of pectic enzymes: a review. Process Biochem. 1998;33(1):21-8.
13. Wilkins M, Widmer W, Grohmann K, Cameron R. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour Technol. 2007;98(8):1596-601.
14. Saleem Z, Rennebaum H, Pudel F, Grimm E. Treating bast fibres with pectinase improves mechanical characteristics of reinforced thermoplastic composites. Composites Sci Technol. 2008;68(2):471-6.
15. Rodriguez-Nogales J, Ortega N, Perez-Mateos M, Busto MD. Pectin hydrolysis in a free enzyme membrane reactor: An approach to the wine and juice clarification. Food Chem. 2008;107(1):112-9.
16. Jayani R, Saxena S, Gupta R. Microbial pectinolytic enzymes: A review. Process Biochem. 2005;40(1):2931-44.
17. Reyes N, Rivas-Ruiz I, Dominguez-Espinosa R, Solís S. Influence of immobilization parameters on endopolygalacturonase productivity by hybrid Aspergillus sp. HL entrapped in calcium alginate. Biochem Eng J. 2006;32(1):43-8.
18. Sartoğlu K, Demir N, Acar J, Mutlu M. The use of commercial pectinase in the fruit juice industry, part 2: Determination of the kinetic behaviour of immobilized commercial pectinase. J Food Eng. 2001;47(4):271-4.
19. Pifferi PG, Spagna G, Busetto L. The immobilization of endopolygalacturonase on γ-alumina. J Mol Catal. 1987;42(2):137-49.
20. Lei Z, Bi S. The silica-coated chitosan particle from a layer-by-layer approach for pectinase immobilization. Enzyme Microb Technol. 2007;40(5):1442-7.
21. Lei Z, Bi S,Yang H. Chitosan-tethered the silica particle from a layer-by-layer approach for pectinase immobilization. Food Chem. 2007;104(2):577-84.
22. Romero C, Sánchez S, Manjon S, Iborra JL. Optimization of the pectinesterase/endo-D-polygalacturonase coimmobilization process Enzyme Microb Technol. 1989;11(12):837-43.
23. Lozano P, Manjon A, Iborra JL, Canovas M, Romojaro F. Kinetic and operational study of a cross-flow reactor with immobilized pectolytic enzymes. Enzyme Microb Technol. 1990;12(7):499-505.
24. Gómez L, Ramírez HL, Villalonga ML, Hernández J, Villalonga R. Immobilization of chitosan-modified invertase on alginate-coated chitin support via polyelectrolyte complex formation. Enzyme Microb Technol. 2006;38(1-2):22-7.
25. Smidsrød O. Solution properties of alginate. Carbohydr Res. 1970;13(3):359-72.
26. Grasdalen H, Larsen B, Smidsrød O. A. p.m.r. study of the composition and sequence of uronate residues in alginates. Carbohyd Res. 1979;68(1):23-31.
27. Baxter A, Dillon M, Taylor KD, Roberts GA. Improved method for i.r. determination of the degree of N-acetylation of chitosan. Int J Biol Macromol. 1992;14(3):166-9.
28. Bernfeld P. Amylases α and β. Methods Enzymol. 1955;1:149-58.
29. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54.
30. Busto MD, Garcia-Tramontin KE, Ortega N, Perez-Mateos M. Preparation and properties of an immobilized pectinlyase for the treatment of fruit juices. Bioresour Technol. 2006;97(13):1477-83.
31. Spagna G, Pifferi PG, Tramontini M. Immobilization and stabilization of a pectinlyase on synthetic-polymers for application in the beverage industry. J Mol Catal A-Chem. 1995;101(1):99-105.
Received in April, 2012.
Accepted in November, 2012.
Hector L Ramirez. Centro de Tecnología Enzimática, Universidad de Matanzas Camilo Cienfuegos, Autopista a Varadero, Km 3½, CP 44740, Matanzas, Cuba. E-mail: hlrperez2003@gmail.com , hector.ramirez@umcc.cu.