Introduction
Uretero-arterial fistula (UAF) is a rare but potentially life-threatening cause of hematuria. 1).Since first described in 1908 by Moschowitz 2 about 160 cases have been reported in the literature. Aging population, the extensive multimodal cancer treatments improving life expectancy of patients with pelvic malignancies, and the use of ureteral indwelling stenting are increasing the incidence of this condition. 3
UAF appears due to different vascular and non-vascular factors that affect the arterio-ureteral crossing 4 causing this communication between artery and urine outflow, with hematuria as the main symptom. It must be suspected in patients with gross intermittent hematuria and a history of arterial wall disease, prolonged ureteral stenting and prior pelvic surgery, malignancy or radiation.
Diagnosis is difficult and imaging rarely demonstrate the fistulous tract 5, so clinical awareness is the most important of all diagnostic steps. The precision and the time to diagnosis are crucial prognostic factors and can cause mortality to vary between 7 to 23 %. 6
Several treatment modalities have been described, which are endoscopic, surgical, endovascular or combination in the context of a multidisciplinary team.7 In the latest years, stent-grafting seems to be the gold-standard but follow-up is still short.
We present a case of massive hematuria and hemorrhagic shock due to a left uretero-iliac artery fistula in a patient with a history of ureteral stenting for stone disease and an aorto-iliac vascular graft, successfully managed with angiography and simultaneous endovascular stent.
Case presentation
We present a case of a 78-year-old-man undergoing low risk non-muscle-invasive bladder cancer with no relapse since 2012 and benign prostatic hyperplasia treated with tamsulosin-dutasteride.
The patient had a history of hypertension, occlusive arterial disease and aorto-bifemoral bypass in December 2014 due to an abdominal aortic aneurysm.
In March 2018 he presented to the Emergency Department with a right flank pain and deterioration of renal function. The ultrasound examination showed a bilateral ureteral obstruction hydronephrosis due to bilateral distal ureteral lithiasis. The patient was taken to the operating theatre for a bilateral ureteroscopy (URS), but during the procedure a bladder calculi caused by the enlargment of the prostate was found making the URS difficult, so a bilateral ureteral stenting was performed. The renal function normalized and three months later we practiced a cistolithotripsy and a transurethral resection of the prostate.
In August 2018 a right URS was successfully performed solving the right distal ureteral lithiasis. However in the left side a 5 mm impacted stone at the level of the arterio-ureteral crossing and subsequent ureteral stricture did not allow the ureteroscope progression, so the ureteral stent was replaced.
Two months later the patient was admitted to hospital for a second attempt of left URS. At this point he had been carrying the left ureteral stent for 9 months, had no flank pain and only referred recent episodes of mild intermittent hematuria. Again a failed attempt of left URS was performed, not being able to progress above the stone that looked epithelized this time. The bladder was also explored and recurrence of bladder tumor as a cause of hematuria was ruled out. Since the patient felt asymptomatic and due to the great morbidity to major surgery, therapeutic abstention for left ureteral lithiasis was decided.
Three days later, after removal of ureteral stent, the patient presented an episode of gross hematuria and flank pain with hemodynamic instability that resolved spontaneously. The CT images showed the already known ureteral distal lithiasis and subsequent hydronephrosis with bladder clots. A 3-way urethral catheter with continuous irrigation was inserted. The following days he continued to have intermittent visible haematuria which intensified four days later, when the patient suffered massive bleeding through the urethra with subsequent hypovolemic shock. Hemoglobin level dropped to 5 g/dL requiring transfusion of 6 units of blood.
At this point and with high suspicious of uretero-arterial fistula, taking into account the patient history and clinical presentation, a CT scan with angiography was urgently performed. As shown in Figure 1 and Figure 2, it was suggestive of fistulous communication between left distal ureter at the level of the lithiasis and the left common iliac artery by-pass, with surrounding inflammatory changes, although the fistulous trajectory could not be demonstrated. It also showed a left-sided uretero-hydronephrosis due to the lithiasis, and blood clots within the left collecting system and the ureter.
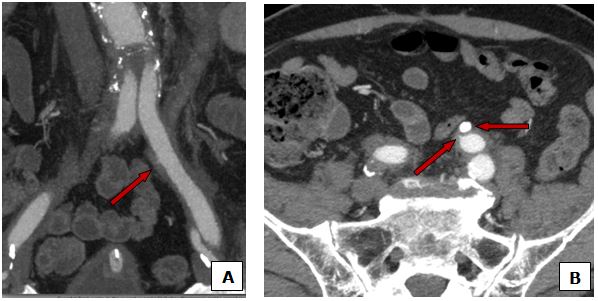
Fig. 1 Inflammatory changes surrounding the left common iliac branch of the bypass with spiculation (A, B arrow) of the contrast in the middle part suggesting fistulous trajectory into the homolateral ureter al the level of the lithiasis (B arrowhead).
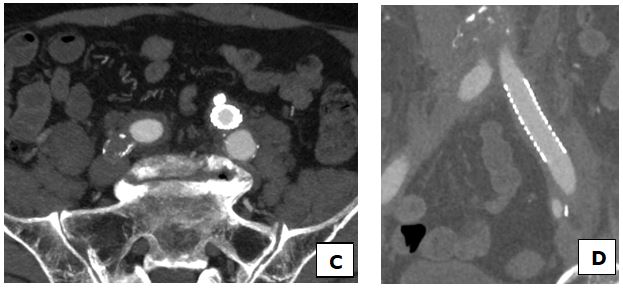
Fig. 2 Resolution of the fistula with the covered iliac common artery stent (C, D). MPI-CT data reconstruction images.
Faced with this observation and when the patient was hemodynamically stable, he was sent to Interventional Radiology for endovascular treatment approach. In collaboration with vascular surgeons, a femoral 9 Fr arteriotomy under local anesthesia was performed and through a left retrograde common femoral approach Angiography study was performed with adapting bolus chasing CT (Siemens Axion Artis System). After 60 ml of Iodixanol infusion, images confirmed the presence of an uretero-prosthetic fistula between the left common iliac branch of the aorto-bifemoral by-pass and the homolateral ureter.
The treatment consisted of implantation of a balloon-expandable covered stent (FluencY® Plus Vascular Stent Graft10 x 60 mm) in the left iliac branch. Angiographic control at the end of the procedure showed the tapering off of the flow of the UAF (Figure 3).
The clinical course was rapidly favorable with the resolution of the hematuria, being the patient discharged 3 days after the procedure.
One year later the patient continues to be monitored for his bladder cancer, currently being asymptomatic.
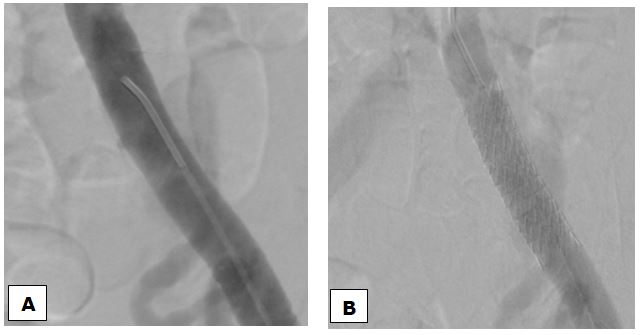
Fig. 3 Images obteined by Digital Substraction Angiography (DSA). Opacification of the left primitive iliac artery shows pseudoaneurysm (A) arising from the middle part of the common iliac artery at the level of the ureteral crossing. Final opacification after implantation of a covered stent showing the tapering off of the flow of the AUF (B).
Discussion of the case
UAF is a rare condition that occurs in the setting of a fistulous communication at the junction were artery crosses ureter. They mainly concern iliac axes with preferential involvement of the external and the common iliac artery, but can involve also the terminal aorta. 8
A variety of medical conditions may predispose to the fistula formation; depending on vascular or non-vascular factors, UAF are classified into primary (15 %) -when they are caused by natural diseases that affect arterial system (aneurysms, vascular malformations, etc)- or secondary (85 %) -when there is a history of abdomino-pelvis cancer (mainly extensive surgery for urological or gynaecological cancer), vascular surgery (graft stenting), radiotherapy and ureteral stenting- 9) They all seem to have contributed to an incidence increased in the latest years, specially linked to the possibility of ureteral stenting since 1978. 10
Though the exact mechanism of the fistula formation is still uncertain it is well known that UAF arise when predisposal risk factors occur. It has been suggested that inflammatory reactions aftersurgery to the ureter wall, local fibrosis of the retroperitoneum after radiotherapy or vascular surgery in the pelvis area can fix the ureter and the artery, surrounded by the inflammatory process. This results in a weakening of the adventitia and media of the artery, increasing their susceptibility to rupture. Thus, high pressures and chronic pulsations to the fibrosed and less compliant ureteral wall can cause necrosis and eventually formation of a fistula. 11
We believe in our patient several risk factors converged such as prior vascular reconstruction surgery of the aorto-iliac axis with synthetic graft and the stone pressure at the level of the arterial-crossing. This probably played an important role in predisposing to local damage and ischemia that added to the repeated URS in a short time and the requirement of prolonged ureteral stent, could lead to the fistula formation. Urine leakage after URS is probably responsible for the intense fibrotic inflammatory response that fixed the ureter to the vascular graft.
Commonest presenting symptom includes hematuria (up to 62 %), ranging from intermittent to life-threatening hemorrhage with severe hypotension or shock. 12 Less common is flank pain due to clot formation in the ureter that eventually occlude the communication between the ureter and iliac artery during quiescent periods. After degeneration of the clot by proteolytic enzymes the bleeding recurs. 13
Diagnosis is difficult and represents a real challenge. Thus, clinical suspicious is essential making mortality rates range from 7 % to 23 % when condition goes undiagnosed. 6 In our patient the magnitude of the hematuria and his vascular and ureteral history clearly made us suspect of the presence of an UAF, which was crucial given the life-threatening situation.
UAF traditionally have been diagnosed with surgical exploration, which otherwise leads to an increased morbidity and mortality.14 However different techniques can be used before surgery to diagnose the fistula, the problem is that imaging studies usually do not visualize the fistula unless active hemorrhage is present.
CT Scans have low sensitivity (42 %) since they are usually negative or non-specific, because they show bleeding only rarely and the fistulous communication is almost never seen. Nevertheless some findings should make us suspect the presence of a fistula, including pseudoaneurysms, signs of graft infection and uretero-hydronephrosis. 15 In our patient the CT scan showed blood clots in the collecting system and ureter and a spiculation of the contrast in the left common iliac artery as an indirect sign of fistula.
Cistoscopy may evidence a pulsatile massive bleeding to one of the ureteral orifices and combined with retrograde pyelography has a sensitivity of 45-60 % and so was the preferred diagnostic technique in the past. 13 Today they are not recommended, neither is ureteroscopy since it can dislodge a tamponanding clot or tear the fistula, leading to massive hemorrhage. 12 In recent years selective iliac arteriography has demonstrated to be the most sensitive technique (69 %) and remains the most important diagnostic tool when AUF is suspected, particularly when we use “provocative maneuvers” manipulating the ureteral stent to provoke active bleeding of the fistula. 16,17 These maneuvers should be attempted only with appropriate surgical backup to handle sever hemorrhage.
Treatment of a diagnosed or suspected UAF requires a multidisciplinary team composed of urologist, vascular specialist and interventional radiologist. Classically open surgery was considered the first line treatment 18; it required the ligation of the involved artery, with or without bypass revascularization, and patch graft in combination with urinary diversion, nephrostomy or nephoureterectomy. Today endovascular therapy has become the mainstay in the management of UAF, proven not only to be effective but also less invasive and quick in this emergency condition. 8,19
They assure the complete closure of the fistula and blood flow anterograde maintenance through the iliac artery, but also avoid the need for direct arterial or ureteral surgery and the need for subsequent revascularization procedures of the lower extremity. The new polytetrafluoroethylene-covered self-expanding stents show very good results, however long-term follow-up results have not been reported yet. 20. In our patient this endovascular approach made possible the fistula exclusion in a short time without compromising the vascular supply.
Final considerations
Uretero-arterial iliac fistula represents a clinical, diagnostic and therapeutic challenge, this is crucial for prompt diagnosis and treatment, otherwise it can became a life-threatening condition. Angiography yields de highest diagnostic benefit while treatment requires a multidisciplinary team composed of urologist, vascular specialist and interventional radiologist.Endovascular therapy is the treatment of choice, increasing the chances of survival of these high-risk patients in a safe and minimal invasive way.














