INTRODUCTION
Neurological disorders are a group of diseases of the Central Nervous System (CNS), which occur because of damage to the brain, or spinal cord. Its main causes are trauma, infections, degeneration, autoimmune conditions, structural defects, tumors, and strokes. The main risk factor associated with these causes is aging 1).
According to reports from the World Health Organization, more the 70% of deaths are due to noncommunicative diseases (NCD) and nearly 4% due to neurological diseases. In addition, 15 million people suffer from stroke every year and 5 million die because of it.2 In Cuba, strokes were the fourth leading cause of death in 2021, causing 12,759 deaths; dementia and Alzheimer's disease ranked sixth in mortality (6,208 deaths), and Parkinson's disease was in the 15th position (933 deaths) 3.
Current treatments do not satisfy the complex management of CNS diseases, so the scientific community researches to develop new, safer, and more effective therapeutic alternatives (1). In recent years, several compounds with neuroprotective potentials have been evaluated, some of which have other pharmacological applications; an example that stands out is erythropoietin (EPO).
EPO is a glycoprotein hormone, which belongs to class I cytokines. Most of it is made by the kidney in response to hypoxia. Its main function is the stimulation of hematopoiesis and the maintenance of blood homeostasis. However, with the finding of the expression of EPO and its receptor in non-hematopoietic tissues, this system is given a new role in tissue protection 4,5.
It has been shown that the expression of EPO receptors is increased in injured tissues, prior to the increase in local production of EPO, so that the administration of exogenous EPO could accelerate innate cytoprotective mechanisms (5. The effectiveness of recombinant human EPO (rhuEPO) as a neuroprotector has been demonstrated in several studies 6,7, but unwanted stimulation of hematopoiesis has limited its use for these purposes.
In recent decades, variants of rhuEPO have been developed without hematopoietic activity, but which retain cytoprotective effects (4, 5). One of these variants is known as NeuroEPO, obtained by the Center for Molecular Immunology in Havana, Cuba8). NeuroEPO has shown protective effect in diabetes 9,10 and neurological diseases 11-14. Its neuroprotective effect has been evaluated in several preclinical studies and clinical trials. The objective of this review is to evaluate the neuroprotective potential of NeuroEPO in neurological disorders.
MATERIAL AND METHODS
A systematic review of scientific articles reporting research results with NeuroEPO was conducted, guided by the question: Is NeuroEPO a safe and effective therapeutic candidate for neuroprotection in neurological disorders?
The sources of information were consulted in the period May-August 2023, using PRISMA guidelines. Because NeuroEPO is a newly produced molecule, the search was not restricted to a specific year period. We proceeded to consult sources of information in the databases LILACS, PubMed and the search engine of Google Scholar. For the correct use of the search terms, the Descriptors in Health Sciences (DeCS/MeSH) were consulted. The terms used in the search were: “NeuroEPO”, “neuroprotection”, “neurological diseases”. The Boolean operator AND was used to combine the terms in the advanced searches.
The corresponding search syntaxes are listed below:
LILACS: NeuroEPO [Words] and neuroprotection [Words] and not ("REVIEW") or "META-ANALYSIS" [Publication type]
PUBMED/MEDLINE: (((((NeuroEPO) AND (Neuroprotection)) NOT (review [Filter])) NOT (systematic review [Filter])) NOT (meta-analysis [Filter])) NOT (books docs [Filter])
An advanced search was carried out in Google Scholar in which the articles were filtered according to criteria and keywords combined with the AND Bolean operators.
The inclusion criteria consisted of original articles, in Spanish or English, from preclinical studies and clinical trials that evaluated the neuroprotective potential of NeuroEPO in CNS diseases. Exclusion criteria were applied to articles that were not available in full text, review articles, and articles with a poorly reproducible methodology.
The articles that were included were subjected to a technical reading (reading of the title and abstract), followed by a deep reading (reading of the full-text version of the article). Each selected article identified: study objective; route of administration, frequency and duration of intervention; main outcomes; main conclusions. In the articles that provide information about preclinical studies, the animals and biomodels used were also identified.
RESULTS
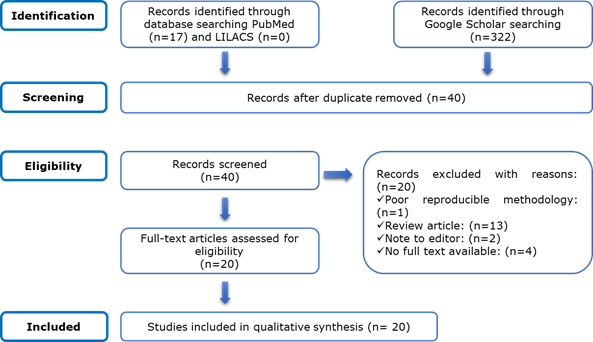
With the search strategy followed, a total of 339 articles were identified from Lilacs (n=0), Pubmed/Medline (n=17) and Google Scholar (n=322). After the elimination of duplicates and the application of the inclusion and exclusion criteria, 20 articles were included for synthesis and review (Figure 1).
To organize the information obtained from the articles and their relevance, tables were prepared in which preclinical studies 11-23) (Table 1) and clinical trials 24-30) (Table 2) were grouped.
Preclinical studies
Preclinical studies (n=13) consisted of in vitro and in vivo experiments (Table 1).
Table 1 Preclinical studies of the neuroprotective potential of NeuroEPO
| Objective of the use of NeuroEPO | Animal/ Biomodel | Route, dose, frequency and duration | Main results | Key findings | Year / Ref |
|---|---|---|---|---|---|
| To demonstrate whether IN reaches the brain and exerts therapeutic efficacy after HF. | 1-Gerbil of Mongolia. 2-Mongolian Gerbil / Permanent focal CI. | rHu-EPOb IN (1 mg/1 mL) 1-labeled with I 125: 100-120 μL. Single dose. 2-10 μg/ 3V/ D/ 4 D. | 1-Radioactivity in olfactory bulb and cerebellum. 2-Lower mortality, clinical signs of HF and tissue damage; better functional integrity. | IN EPO reach the brain and exerts neuro-protective effects. | 2006 11 |
| To demonstrate the arrival to the brain when administered via IN. | 1-Gerbil of Mongolia. |
rHu-EPOb I125 IN (1 mg/1 mL) single dose: 1-100-120 μL. 2-1 mg/1 mL. | 1-Radioactivity in olfactory bulb and cerebellum. 2-Radioactivity in CSF. | The IN pathway is an alternative with quick access to the brain. | 2008 12 |
| To evaluate the neuroprotective effect in HF. | Wistar rat/ transient focal IC. | rhu-EPOb IN (1 mg/1 mL): 10 uL) / 3 V/ D/ 2 D. | Neurological improvement; reduced area of infarction; slight histological alterations in hippocampus. | Neuroprotective effect. | 2009 (13) |
| To determine the neuro-protective effect compared to rHu-EPO IP in HF. | Mongolian Gerbil / Permanent focal IC. | NeuroEPO IN: 10 μg (249.4 IU)/ 3 V/ D/ 4 D. -rHu-EPO IP: 5000 IU/ 3 V/ D/ 4 D. | Lower mortality; improved neurological status and cognitive function; CA3 protection of hippocampus, temporal cortex and thalamus. | Better neuroprotective effect than rhu-EPO. | 2010 (14) |
| To evaluate the toxicological potential of the nasal formulation. | Wistar Rat. | rhEPOb IN: 22,000 IU/ml (0.7 mg/ml) 1-330 IU. Single dose (NID). 2-47.143 UI/kg in 6 h (ETA). 3-6,600 IU/kg/ D/ 14 D (ETSA). | 1-Minimal edema and nasal vascular congestion. 2-Hematological variables, body weight and organs without alterations. 3- Reversible changes in the nasal mucosa, without alterations of other tissues and organs, nor adverse hematological events. | It may offer the same neuro-protection as EPO, without hematological side effects. | 2011 (15) |
| To evaluate the neuroprotective effect in HF. | Mongolian Gerbil / Permanent focal IC. | NeuroEPO IN: 10 μL/ 3 V/ D/ 4 D. | Lower mortality and neuronal loss; improved motor and sensory function; different expression patterns of Ngb and EPOR in cerebral cortex and hippocampus. | It is effective, with a close relationship of EPO/EPOR and Ngb in neuro-protection. | 2011 (16) |
| To determine the effect of dose and therapeutic window on HF. | Mongolian Gerbil / Permanent focal IC. | NeuroEPO IN: 10 μL (249, 4 IU) / 3 V/ D/ 4 D. | Lower mortality; improved neurological status; CA3 protection of hippocampus, cortex and thalamus. | Up to 12 h after HF is very positive as neuro-protective. | 2012 (17) |
| To confirm the protective activity and compare it with that induced by rHu-EPO in AD. | Swiss / non-transgenic EA mouse. | NeuroEPO IN: 62, 125 and 250 μg/kg/ 3 V/ D/ 4 D. -rHu-EPO IP: 125, 250 and 500 μg/kg/ D/ 4 D. | Both EPOs prevented: learning deficit, cell loss, lipid peroxidation, increase in Bax, TNFα and IL-1β, and decreased activation of Akt, in hippocampus. | Neuroprotective activity of both EPO. | 2013 (18) |
| To analyze neuroprotective activity in AD. | EASwe/transgenic APP mouse. | NeuroEPO IN: 125 and 250 μg/kg/ 3 V/ D/ 3 D/ S/ 2 M. | Less neurological deficit and memory alterations; lower lipid peroxidation, Bax/Bcl-2, TNFα, FasL and amyloid deposition in hippocampus and cortex. | Neuroprotective activity, by reduction of oxidative stress, neuroinflammation, apoptosis and amyloid deposits. | 2017 (19) |
| Determine how it protects neurons from EIG. |
|
NeuroEPO: 10-100 ng/mL medium/ 24 h. | Neurons with lower mortality, morphological changes and total oxidant activity, and greater total antioxidant capacity. | Neuroprotective effect, by reducing oxidative stress. | 2018 (20) |
| To analyze the effect against neuronal apoptosis by EIG. |
|
NeuroEPO: 100 ng/mL medium/ 24 h. | Neurons with lower mortality, morphological changes, release of cytochrome c, and expression and activity of caspase 3, and increase of Bcl-2/Bax. | Protection of cortical neuron apoptosis. | 2018 (21) |
| To determine the effect of the nasal formulation on the olfactory mucosa and lymphatic tissue. | Wistar Rat. | NeuroEPor IN: 300 μg/kg/ D/ 28 D. | Absence of alterations in the respiratory mucosa and associated lymphatic tissue. | It does not cause histopathological changes in respiratory mucosa and its lymphatic tissue. | 2022 (22) |
| To determine the effect of the nasal formulation on the olfactory mucosa. | Wistar Rat. | NeuroEPO IN: 300 μg/kg/ D/ 28 D. | In the absence of alterations of olfactory epithelium; slight hypertrophy and hyperplasia of Bowman's glands. | It does not cause histopathological changes in olfactory mucosa. | 2022 (23) |
Legend: IN- intranasal; IP- intraperitoneal; HF- cerebral ischemia; CSF- cerebrospinal fluid; h- hours; D- day/days; S-weeks; M- months; V- time/times; EIA- nasal irritation assay; ETA- acute toxicity test; ETSA- subacute toxicity test; EPO- erythropoietin; EPOR-erythropoietin receptor; Ngb- neuroglobin; AD- Alzheimer's disease; Bax- pro-apoptotic protein; TNFα- tumor necrosis factor-alpha; IL-1β- interleukin 1 beta; Akt- protein kinase B; Bcl-2- antiapoptotic protein; FasL- Fas ligand; EIG- glutamate-induced excitotoxicity; Stroke- stroke.
In vivo preclinical studies (n=11) investigated the neuroprotective effect of NeuroEPO in biomodels of cerebral ischemia (n=5) and Alzheimer's disease (n=2), as well as the arrival to the brain (n=2) and safety (n=3) of the product administered intranasally (IN). Most studies were conducted in rodents (n=11) and one in the non-human primate Macaca fascicularis. The route of administration in all cases was IN, with a predominance of the use of the 1 mg / 1 mL formulation. Various doses, frequency of administration and duration of the intervention were evaluated. In the main results, with the administration IN of NeuroEPO, it was observed:
minimal local irritation (n=1), and absence of irreversible histopathological alterations of the nasal mucosa (n=3) and associated lymphatic tissue (n=1);
absence of structural alterations in organs and tissues outside the nasal cavity, as well as hematological alterations (n=1);
after administration of the molecule labeled with I125, radioactivity in the olfactory bulb, cerebral cortex (n=2) and cerebrospinal fluid (n=1);
in biomodels of cerebral ischemia, lower animal mortality (n=4), less brain tissue damage and better neurological status (n=5), and changes in EPO receptor expression (EPOR) and neuroglobin (Ngb) and (n=1);
in biomodels of Alzheimer's disease, lower neurological deficit, and brain tissue with less cell loss, lipid peroxidation and proapoptotic and proinflammatory markers, and increased antiapoptotic markers (n = 2) and protein kinase B or Akt activity (n = 1).
compared to rhuEPO administered intraperitoneally, a better neuroprotective effect was found in cerebral ischemia (n = 1), similar to Alzheimer's disease (n = 1).
In vitro studies (n=2) were aimed at investigating neuroprotection in a model of stroke, in primary culture of neurons from Wistar rat embryos. When adding NeuroEPO to the culture, a decrease in cell mortality and morphological changes caused by glutamate-induced excitotoxicity was observed. The results included lower oxidant activity and greater antioxidant capacity (n = 1), as well as decreased proapoptotic markers and increased anti-apoptotic markers (n = 1).
In preclinical studies in general, the evaluated product was identified as either NeuroEPO (n=5), rHu-EPOb (n=3), NeuroEPO (n=3), rhEPOb (n=1) or neuroEPO (n=1).
Clinical trials
We reviewed articles (n=7) reporting results of clinical trials of NeuroEPO (Table 2) in healthy volunteers (n=1) and in patients with Parkinson's disease (n=4), spinocerebellar ataxia type 2 (n=1) or Alzheimer's disease (n=1). The product was identified as NeuroEPO (n=6) or neuroEPO (n=1). In all studies, the NI route and the 1 mg dose were used; two studies also evaluated the 0.5 mg dose. The frequency of administration of the product and the duration of the intervention were variable. In the main results, with the NeuroEPO IN it was observed:
in all studies assessing safety and tolerability, predominance of mild adverse events, which resolved without treatment, and absence of hematopoietic abnormalities (n=3);
In all studies evaluating the efficacy of the intervention (n=5), clinical improvement of patients.
Table 2 Clinical trials of the neuroprotective potential of NeuroEPO
| Objective | Route, dose, frequency and duration | Main results | Key findings | Year (ref) |
|---|---|---|---|---|
| To assess the safety of NeuroEPO in healthy volunteers. RPCEC00000157, 10 June 2013. | NeuroEPO IN (1 mg/ 1 mL): 0.5 and 1 mg/ 8 h/ 4 D. | Predominance of mild ADVs, which resolved without treatment; no hematological alterations. | Safe product, well tolerated at the level of the nasal mucosa, without stimulation of erythropoiesis. | 2017 (24) |
| To show the neuro-psychological changes of PD patients treated with neuroEPO (states 1-2 H and Y) www.clinicaltrials.gov NCT04110678) or EPOrh. | 1-EPOrh SC: 60 IU/kg 1 V/ S/ 5 S. 2-neuroEPO IN (1 mg/1 mL): 1 mg/ 1 V/ S/ 5 S. | Improved cognitive function with both EPOs. | Both EPOs have a positive effect on cognitive function. | 2018 (25) |
| To evaluate the short-term tolerance of NeuroEPO in patients with PD (states 1-2 H and Y). www.clinicaltrials.gov NCT04110678 | NeuroEPO IN (1 mg/1 mL): 1 mg/ 1V/ S/ 5 S. | Mild, brief ADVs that did not require treatment and left no sequelae. | Nasal administration is well tolerated for 5 weeks. | 2021 (26) |
| To assess whether NeuroEPO improves cognitive function in patients with PD (states 1-2 H and Y). www.clinicaltrials.gov NCT04110678 | NeuroEPO IN (1 mg/1 mL): consecutive 1-1 mg/ 1 V/ S/ 5 S. 2-1 mg/ 3 V/ S/ 1 M. 3-0.5 mg/ 3 V/ S/ 8 M. | Direct and significant effect of dose on cognitive status, with positive influence of educational level and negative influence of age. | Positive effect on cognitive status, with greater benefit for the youngest and with a higher educational level. | 2022 (27) |
| To assess whether NeuroEPO has a positive influence on the cognitive status of patients with PD (states 1-2 H and Y) and whether the effect is reflected in qEEG. www.clinicaltrials.gov NCT04110678 | NeuroEPO IN (1 mg/1 mL): 1 mg/ 1 V/ S/ 5 S. | Improvement of cognitive status; cognitive effects reflected in qEEG. | Positive influence on cognitive status and a large portion of the effects is mediated by brain mechanisms that are reflected in the qEEG. | 2022 (28) |
| To investigate the safety, tolerability and clinical effects of NeuroEPO in patients with SCA2. https://rpcec.sld.cu/ensayos/ RPCEC00000187-SP | NeuroEPO IN: 1 mg/3 V/ S/ 6 M. | Absence of severe EAdv; increased motor performance; decreased latency of saccadic shakes; absence of hematopoietic alterations. | Safety and tolerability were demonstrated, with a small beneficial effect on motor and cognitive abnormalities. | 2022 (29) |
| To assess the efficacy of NeuroEPO in reducing the progression of cognitive impairment in patients with mild/moderate AD. RPCEC00000232, February 01, 2017 | NeuroEPO IN: - 0.5 mg/ 3 V/ S/ 48 S. - 1 mg/ 3 V/ S/ 4 S, followed by 0.5 mg/ 3 V/ S/ 44 S. | Cognitive and psychobehavioral improvement. | Efficacy in reducing the progression of cognitive impairment. | 2023 (30) |
Legend: RPCEC- Cuban Public Registry of Clinical Trials; IN- intranasal; SC- subcutaneous; h- hours; D- days; S-weeks; M- month/months; V- time/times; EAdv- adverse events; PD- Parkinson's disease; H and Y- Hoehn and Yahr scale; EPO- erythropoietin; qEEG- quantitative electroencephalogram; SCA2- spinocerebellar ataxia type 2; EA- Alzheimer's disease.
DISCUSSION
Neuroprotection is an essential purpose in the treatment of neurological disorders. In recent decades, several investigations have been aimed at evaluating the neuroprotective potential of NeuroEPO in preclinical or clinical trials. Their results show that IN administration did not produce local structural damage 15,22,23 or outside the nasal cavity 15), nor hematological alterations 15,24,29 or serious adverse events, and was well tolerated by patients (24,26,29. After IN administration, it reached the brain 11,12 and exerted neuroprotective effects in biomodels of cerebral ischemia 11,13,14,16,17 and Alzheimer's disease 18,19, as well as in patients with Parkinson’s disease 25,26,27,29, spinocerebellar ataxia type 2 29 or Alzheimer's disease 30. Neuroprotective effects were manifested by decreased neuronal mortality and damaged tissue 11,13 21, as well as improved functional integrity 11,13,14,16-19. Molecular neuroprotection mechanisms included changes in expression and activity of EPOR, Ngb 16 and Akt 18, and decreased oxidative stress 18-20)., inflammation (18,19 and apoptosis 18,19,21.
Stroke and neurodegenerative diseases are common causes of disability and mortality at the world level (1-3, 31,32; of these, ischemic type is the most common stroke 31, while Alzheimer's and Parkinson's are the most common neurodegenerative disorders 32. On the other hand, spinocerebellar ataxia type 2 is a hereditary neurodegenerative disease, with low prevalence worldwide, but Cuba has a high concentration of patients and offspring at risk, especially in the province of Holguín 33). The statistics and their impact justify that all the studies included in this review evaluated NeuroEPO in these types of conditions.
Although there was a predominance of preclinical studies demonstrating beneficial effects of NeuroEPO in cerebral ischemia, all clinical trials reviewed correspond to neurodegenerative diseases. This result coincides with that reported in the literature that few clinical studies provide comprehensive and up-to-date knowledge of the usefulness of EPO for the treatment of ischemic stroke (6).
NeuroEPO is a relatively young molecule and there are few articles reporting research results in Alzheimer's disease, and only one in patients with spinocerebellar ataxia type 2. In addition, the results found from the evaluation of NeuroEPO in Parkinson's disease belong to studies in patients included in the same clinical trial (www.clinicaltrials.gov NCT04110678). This limits the number of patients with neurodegenerative diseases in whom the product has been evaluated. However, the evidence of the neuroprotective effects of NeuroEPO is relevant from the clinical point of view, which supports the development of additional clinical trials in neurodegenerative diseases, given its personal, family and social repercussions.
Although the product evaluated in the reviewed studies was identified in various ways, its description allows us to recognize in all cases the NeuroEPO obtained by the Center for Molecular Immunology of Cuba. This molecule has a low content of sialic acid 34), which makes it similar to EPO produced in the CNS 14. But the main attraction of this feature is that the low content of sialic acid reduces the half-life of the molecules in the plasma, which means that the unwanted effects of hematopoiesis stimulation are avoided 34, as demonstrated in studies included in the present review (15, 24, 29). These results, and the absence of secondary local or systemic damage (15,22-24,26,29, demonstrate the safety of the administration of intranasal NeuroEPO at the doses evaluated.
On the other hand, the more rapid degradation of hyposialic forms of EPO, makes the administration of IN NeuroEPO an advantage over systemic routes of administration to achieve CNS effects. This, together with the evidence of safety and tolerability 15,22-24,26,29, as well as the rapid arrival of the molecule in the brain11,12, have made IN administration the route of choice in the evaluation of NeuroEPO as neuroprotective.
One of the fundamentals of the use of NeuroEPO in neuroprotection is the expression of EPO receptors in brain tissue 6,7). In its hematopoietic action, EPO binds to a receptor (EPOR) that forms homodimers in the cell membrane, while cytoprotective effects require the activation of a heterodimers-receptor, composed of EPOR and the common β cytokine receptor (βcR), also known as CD131 35. Changes in the expression of EPOR were identified when administering NeuroEPO in a biomodel of cerebral ischemia, as well as the endogenous neuroprotective neuroglobin (Ngb) 16), with patterns suggesting that the EPO/EPOR system and Ngb are closely linked to neuroprotection.
It is known that the binding of EPO to its receptors stimulates the autophosphorylation and activation of JAK2 (Janus kinase 2), thereby triggering three intracellular signaling pathways: the signal transducer and transcription activator pathway (STAT), the phosphatidylinositol-3-kinase/Akt pathway or protein kinase B (PI3K/Akt), and the mitogen-activated protein kinase (MAPK) cascade (4,5. These pathways are important regulators of cell differentiation, proliferation, and survival, and mediate the cytoprotective effects of EPO 4,5,36).
Several studies have shown that the pathways activated by EPO have anti-apoptotic, anti-inflammatory and antioxidant effects, which mediate neuroprotection 6,7,37. Oxidative stress and neuroinflammation are different pathological events, but they are closely connected with the mechanisms of tissue damage, both in ischemic stroke 38 and in neurodegenerative diseases 39).
In studies with NeuroEPO in biomodels of Alzheimer's disease, a decrease in neuronal loss, activation of Akt, decreased oxidative damage to lipids and pro-inflammatory and proapoptotic markers, and increased antiapoptotic markers were observed (18,19). Likewise, in an in vitro model of stroke, administration of NeuroEPO increased cell viability, total antioxidant capacity, and antiapoptotic markers, and decreased total oxidant activity and pro-apoptotic markers 20,21). The results suggest that NeuroEPO exerts neuroprotective effects by reducing oxidative stress, inflammation and apoptosis.
CONCLUSIONS
NeuroEPO has proven to be a safe product, without hematopoietic effects, and well tolerated intranasally, which provides benefits in situations of ischemic or degenerative brain damage, by stimulating endogenous neuroprotection mechanisms with antioxidant, anti-inflammatory and anti-apoptotic action. The results support the continuation of studies aimed at enriching the scientific evidence of the potential of NeuroEPO for the treatment of neurological disorders.