INTRODUCTION
The erect prickly pear (Opuntia stricta Haw.) is a forage of extreme importance for the Brazilian semi-arid region because it is resistant to prolonged drought periods, being an alternative for animal feeding in times of prolonged drought (Escobar et al., 1986; Barbosa et al., 2018). The Brazilian semiarid is characterized by erratic rains, with long periods of drought and high temperatures (Santos et al., 2017)
Due to some problems in the sexual propagation (Escobar et al., 1986), the production of seedlings of this specie for the distribution in the cooperatives has been done basically through the direct preparation of whole raquettes or through the technique of micro-cleavage, sectioning the cladodes and planting in beds until the development of rackets and transplantation for the field (Gava and Lopes, 2012). However, this technique presents some difficulties, for example, exposure of plant material to the attack of microbial pathogens and physical and temporal spatial limitation for the production of seedlings.
Micropropagation is an in vitro plant culture technique widely used for the production of large scale seedlings in a fast and safe way. This technique allows the production of plants identical to the parent plant, maintaining the genetic identity in short time space and physical environment (Zoghlami et al., 2012; Singh, 2015). Silva and Souza (2017) confirm the effectiveness of the micropropagation for the production of seedlings of O. stricta for field planting.
In micropropagation, growth regulators play an important role in plant development. The cytokinins are a group of growth regulators that are related to the induction of cell division and regulation of the formation of new shoots processes (Cheng et al., 2013; Gentile et al., 2017). The type and concentration of cytokinins used in the culture medium are of great importance for plant regeneration and multiplication during micropropagation (Aremu et al., 2012). Many micropropagation systems use 6-benzylaminopurine (BAP) cytokinin because it is more efficient and economically viable (Werbrouck et al., 1995; Rahman et al., 2018). However, several morphophysiological disorders such as hyperhydricity have been attributed to the use of this regulator.
Hyperhydricity is a morphophysiological disorder in which the plant presents abnormal accumulation of water inside the cells or tissues, which results in a translucent appearance (Vasconcelos et al., 2012). According to some authors (Quiala et al., 2009; Lema-Rumińska and Kulus, 2014; Lázaro-Castellanos et al., 2018), hyperhydricity is a highly relevant problem in the micropropagation of Cactaceae, because it can hinder the acclimatization stage.
In acclimatization it is essential to have seedlings with high growth pattern and uniformity because it will be used to plant new fields. Thus, the morphophysiological quality of the seedlings is extremely important for success in establishing the plants in the field (Silva and Souza, 2017).
The discovery of a new group of aromatic cytokines has been studied as an alternative to use BAP in order to reduce physiological disturbances caused by their excessive or prolonged use, as well as the reduction of oxidative stress in micropropagated plants (Bairu et al., 2009; Amoo et al., 2011; Aremu et al., 2012; Naaz et al., 2019). Considering the abnormal development of O. stricta on in vitro culture, the objective of this research was to evaluate the effect of different types and concentrations of cytokinins on the morphophysiological aspects of in vitro multiplication of O. stricta.
MATERIALS AND METHODS
The experiment and the biochemical analysis were carried out in the Plant Tissue Culture Laboratory (PTCL) at the Federal Rural University of Pernambuco (UFRPE), Recife, Brazil.
Plant material
Shoots of erect prickly pear were obtained in the Biofactory Governador Miguel Arraes of the Center of Strategic Technologies of the Northeast (CETENE) in Recife, Pernambuco, Brazil.
In vitro multiplication
Shoots from Erect prickly pear (O. stricta), in the 2nd subculture, in semisolid multiplication medium containing Murashige and Skoog (1962) (MS) medium supplemented with 30 g l-1 sucrose, 2.22 µM l-1 BAP and 0.57 µM l-1 3-indoleacetic acid (IAA) were transferred to semisolid MS medium without BAP and cultured for 45 days. Explants of approximately 1.5 cm were obtained after excision in the apical and root regions of shoots, and it were used as explants.
The experimental treatments included different concentrations and cytokines types in the multiplication medium, composed by semisolid or liquid MS medium supplemented with 30 g l-1 sucrose and 2.22 or 1.11 µM l-1 BAP + 0.57 µM l-1 IAA or 1.11 µMl-1 6-(3-Hydroxybenzylamino) purine (meta-Topolin, mT) + 0.57 µM l-1 IAA. The control treatment was the medium containing 2.22 µM l-1 BAP and gelled with agar. The pH of the all media was adjusted to 5.8 before autoclaving the liquid medium and before addition of the agar (Agar powder, for microbiology, Sigma-Aldrich) in the semisolid medium.
Two explants were inoculated on a 250 ml capacity vessel containing 20 ml of multiplication medium. At the moment of explants inoculation, the initial fresh biomass was determined with an analytical balance (Bel 120g mode M214Ail, resolution of 0.0001g) and after the period of shoots multiplication. The vessels were kept in growth room at 25±2 °C under cold white light (40 μM m-2s-1) with 16 hours photoperiod over 45 days.
Shoots formation index (SFI), callus formation index (CFI), rooting index (RI) and hyperhydricity index (HI) were determined by dividing the number of responsive explants among the number of total explants. The hyperhydricity index was evaluated according to the occurrence of characteristics translucent and vitreous shoots in each treatment.
Biochemical analysis
With the objective of to evaluate the level of oxidative stress in O. stricta plants in each treatment it were analyzed the enzymatic activity, the content of hydrogen peroxide (H2O2) and malondialdehyde (MDA). Ascorbate peroxidase (APX) and catalase (CAT) activity were determined using 0.1 g plant material that was triturated in liquid nitrogen with the addition ~0.01 g polyvinylpolypyrrolidone. This mixture was homogenized in 1 ml extraction buffer (pH 7.0) containing potassium phosphate, 1,4-dithiothreitol, and ethylenediamine tetra acetic acid. The homogenate was centrifuged at 10 000 g for 20 min at 4°C and the supernatant was collected for analysis. APX activity was determined according to the method proposed by Nakano and Asada (1981) at 290 nm with a 60 seconds interval. It was calculated based on the molar extinction coefficient for ascorbate (2.8 mM-1 cm-1) and expressed as μmol AsA mg-1 protein min-1. CAT activity was determined according to the methodology proposed by Beers and Sizer (1952) at 240 nm from the molar extinction coefficient for hydrogen peroxide (H2O2, 36 mM-1 cm-1), and expressed in μmol H2O2 mg-1 protein min-1.
In the measurement of hydrogen peroxide (H2O2 and malondialdehyde (MDA) contents, 0.2 g of sample macerated in liquid nitrogen was used. Then, approximately 0.02 g Polyvinylpolypyrrolidone (pvpp) and 2 ml trichloroacetic acid (TCA) at 0.1% were added. The plant material was homogenized and centrifuged at 14000 g for 10 minutes at 4°C. The supernatant was used as a plant extract for the analysis. The levels of H2O2 were verified according to methodology proposed by Alexieva et al. (2001) and expressed as μM g-1 FW. The MDA levels were measured according to Heath and Packer (1968) and expressed as nmol g-1 FW.
Experimental design and statistical analysis
The experimental design was carry out in a completely randomized design with six treatments (BAP/2.22, BAP/1.11, mT/1.11, BAP/2.22, BAP/1.11 e mT/1.11) with twelve repetitions. The experimental unit was a vessels containing two explants. The variables analyzed were multiplication rate (determined by dividing the number of responsive explants among the number of total explants), shoot length (cm), fresh biomass (g), enzymatic activity, APX (µmol AsA mg-1 protein min-1), CAT (µmol H2O2 mg-1 protein min-1), H2O2 (µmol g-1 FW) and MDA (nmol g-1 FW).
The statistical analyzes were performed using the ASSISTAT statistical program 7.6 beta. The data obtained were submitted to the Kolmovorov-Smirnov and Bartlett tests for confirmation of normality and variances homoscedasticity at 5% probability level (p<0.05). The ANOVA was performed to determine significant differences between treatments, and when significant were submitted to Tukey test at 5% probability level (p<0.05). Pearson correlations (p<0.05) were performed among all variables.
RESULTS
The consistency in the culture medium significantly influenced shoot formation. The explants cultivated in liquid medium did not present enough shoot multiplication for the biochemical analysis. There was no influence of cytokinin types and concentrations on the in vitro multiplication rate of erect prickly pear in semisolid medium. Nevertheless, shoots formed in medium with mT presented higher fresh biomass length and biomass than explants formed in the BAP treatments (Table 1).
The results of fresh biomass showed a significant positive correlation (r = 0.59, p<0.05) with shoot height. The shoot length was reduced in BAP treatments. Fresh biomass had a tendency to decrease in the treatment with 2.22 μM l-1 BAP.
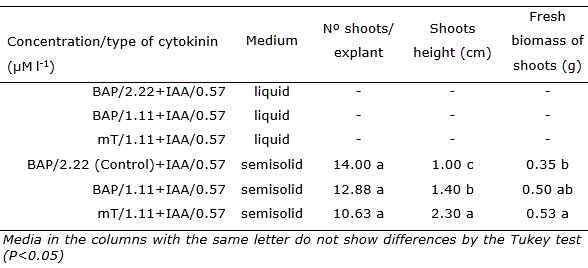
Table 1 Number of shoots per explant, height and fresh shoots biomass in Opuntia stricta Haw. in vitro grown with different types and concentrations of cytokinins in liquid and semisolid medium for 45 days.
The use of mT reduced callus formation by 59% in relation to the control treatment stimulating the rooting and the shoots growth. Although, high concentrations of BAP in semisolid media caused rooting inhibition (Table 2). In all treatments with BAP, roots were formed from the explants. However, in the treatments containing mT, root formation was verified in the shoots (Figure 1).
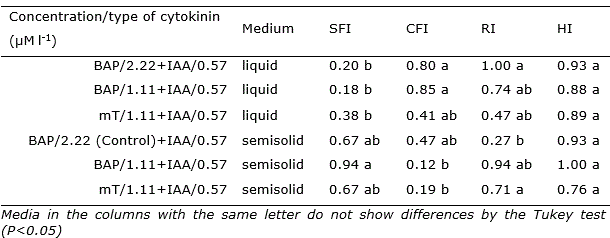
Table 2 Shoots formation index (SFI), callus formation index (CFI), rooting index (RI) and hyperhydricity index (HI) in Opuntia stricta Haw. in vitro cultivated in liquid and semisolid medium with different types and concentrations of cytokinins, during 45 days.
It was observed the occurrence of explants that did not present shoots and showed deformation of the pods, modified leaf bases as found in palm (Areces-Mallea, 2017), especially in explants cultivated with BAP in liquid medium (Figure 1 AB). Many pods fell during the multiplication period and in the areola region there was callus formation, especially in treatments in liquid medium (Figure 1 A-C).
The explants cultured in liquid medium in the presence of mT showed an increase in shoot formation (47 and 52% in relation to the treatments with 2.22 and 1.11 μM l-1 BAP, respectively) (Table 2). Regardless of the consistency of the nutrient medium, it was observed the occurrence of explants with hyperhydricity and callus formation (Table 2). In the semisolid medium with BAP, there was a higher frequency of hyperhydricity in the shoots, unlike the medium with addition of mT, where the branches showed uniformity and vigor (Table 1, Figure 1 DF).
The CAT activity did not differ among the evaluated treatments. Regarding APX, the enzymatic activity was lower in the treatment with 1.11 μM BAP (Table 3). Meanwhile, the highest levels of H2O2 and MDA coincided with the higher incidence of hyperhydricity and possible presence of betacyanin in BAP treatments, especially at high dose (2.22 μM). It was not possible to analyze the enzymatic activity and the contents of H2O2 and MDA in the shoots in liquid medium, mainly due to the low number of shoots and microbial contamination.
Table 3 Activity of ascorbate peroxidase (APX), catalase (CAT), hydrogen peroxide (H2O2) and malondialdehyde (MDA) in shoots of Opuntia stricta Haw. in vitro cultivated in liquid and semisolid medium with different types and concentrations of cytokinins for 45 days.
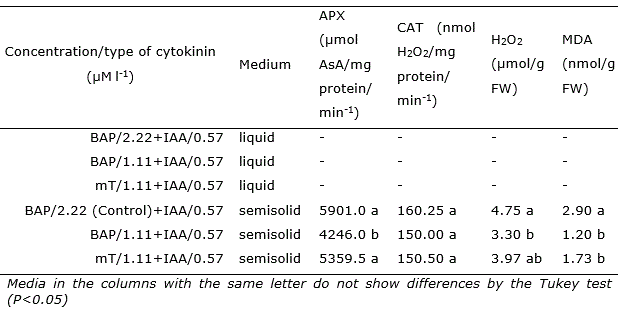
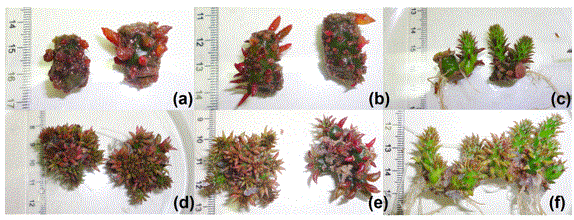
Figure 1 Aspects of Opuntia stricta Haw. explants cultured in vitro with different types and concentrations of cytokinins for 45 days. (a) Explants cultured in liquid medium containing 2.22 μM l-1 BAP. (b) Explants cultured in liquid medium containing 1.11 μM l-1 BAP. (c) Explants cultured in liquid medium containing 1.11 μM l-1 mT. (d) Explants grown on semisolid medium containing 2.22 μM l-1 BAP. (e) Explants grown on semisolid medium containing 1.11 μM l-1 BAP. (f) Explants grown on semisolid medium containing 1.11 μM l-1 mT.
DISCUSSION
The regeneration and multiplication of shoots during micropropagation can be affected by the type and concentration in the growth regulator used, especially the cytokinins, due to their importance in the process of cell division and organogenesis, responsible for inducing a greater number of shoots (Aremu et al., 2017; Abd Aziz et al., 2018). The cytokinin choice to be used in plants micropropagation is determined by their efficiency for inducing the proliferation, formation of healthy shoots, stimulating the acclimatization of plants (Bairu et al., 2007). Since the 1980s, the cytokinins have importance in the in vitro Opuntia (Opuntia amyclaea) multiplication (Escobar et al., 1986). In the present work, the efficiency of mT in shoots formation with superior morphophysiological quality was evident in relation to shoots regenerated in explants cultivated with BAP.
The reduction in shoot length of O. stricta may be related to the toxic effect of BAP on plants, due to the accumulation and the slow release of metabolites to other parts of the plant, causing inhibition of growth and rooting (Erig et al., 2002; Bairu et al., 2007; Aremu et al., 2017). In this sense, Erig et al. (2002) observed a reduction in shoots height and fresh weight of Rubus idaeus L. due to the increase in BAP concentrations.
When O. stricta grown in semisolid medium with 2.22 μM l-1 BAP the shoots showed a rooting inhibition (Figure 1 D). This response may be related to the increase in the levels of the stable metabolite 6-benzyl-amino-9-β-D-glucopyranosylpurine (BAP9G), whose accumulation can cause root growth inhibition and lead to problems in plants acclimatization, according to the results of different investigations (Amoo et al., 2011; Plačková et al., 2015).
As observed in this study, other authors confirmed the efficacy of mT in promoting rooting and reduction losses during the acclimation stage (Valero-Aracama et al., 2009; Aremu et al., 2012). When multiplication and rooting rates are satisfactory (Figure 1 C, Figure 1 F), it is possible to dispense the stage of shoots elongation before acclimatization in the natural environment (Csabai et al., 2011). The increase in the shoots length in medium containing mT has already been described by other authors (Bairu et al., 2007; Gentile et al., 2017; Madzikane-Mlungwana et al., 2017; Naaz et al., 2019).
The excess of cytokinins may favor the occurrence of hyperhydricity, especially in culture with liquid medium with BAP (Figure 1 A-B). Hyperhydricity is considered a morphophysiological disorder that, depending on the intensity, may make the explants recalcitrant (Cruz et al., 2009; Vasconcelos et al., 2011). Among the factors stimulate the hyperhydric formation in shoots are the high humidity and the high concentration of growth regulators (Ziv, 2005; Lebedev et al., 2018). Plants grown in static liquid systems are more susceptible to develop hyperhydricity because are immersed in the culture medium (Adelberg and Naylor-Adelberg, 2012). This condition hinders gas exchange and it can be detrimental to plant cells (Gao et al., 2017).
In addition, several studies have reported a higher frequency of hyperhydricity in a variety of species grown in liquid medium (Tascan et al., 2010; Balen et al., 2012; Camargo et al., 2019). For instance, in the micropropagation of Telekia speciose (Schreber) with 5 μM l-1 BAP or mT were recorded 50% and 5% of hyperhydricity in the shoots development, respectively (Csabai et al., 2011). Amoo et al. (2011) report the effects of different types and concentrations of cytokinins in the Barleria greenii (Balkwill) micropropagation and emphasize the less mT toxic nature in relation to equimolar BAP concentrations, indicating its superiority in producing more shoots with reduction or total absence of abnormalities. Other authors also observed inhibition of shoots formation and a higher rate of abnormal sprouting with increasing BAP concentration, whereas treatments containing mT showed low abnormality rates (Bairu et al., 2007; Wojtania, 2010; Amoo et al., 2011; Aremu et al., 2012). The results obtained in the present research are in agreement with the mentioned authors. These responses suggest that the structural difference between BAP and mT may have a profound impact on micropropagated plants (Bairu et al., 2007), influencing the quality of the seedlings produced and possibly the survival during acclimatization on natural environment (Peixoto et al., 2006).
The occurrence of abnormal characteristics in mT shoots grown (Figure 1 C, Figure 1 F) in the present work may be a consequence of the transition effects between BAP and mT (Amoo et al., 2011), since the explants previously used already had exposed to the BAP. Hyperhydricity is affected by the source and concentration of cytokinin, but the frequency with which the phenomenon occurs is also related to the micropropagated species (Kadota and Niimi, 2003; Liu et al., 2017). According to Amoo et al. (2011), there are still some difficulties in the large-scale production of micropropagated plants, such as low multiplication rate, morphological abnormalities and inadequate rooting. These problems can be minimized by using the appropriate type and concentration of the growth regulator, especially the cytokinins, as they are related to cell division.
In the present work hyperhydric shoots showed intense purple coloration, mainly in the pods (Figure 1 A-B and D-E). A group of non-photosynthetic pigments, the betalains, is subdivided into betacyanins (purple-violet pigments) and betaxanthines (yellow pigments) (Soriano-Santos et al., 2007). Red beet (Beta vulgaris L.) and Opuntia genus are the main sources of these pigments that are used as natural dyes (Georgiev et al., 2008). According to Pavokovi and Krsnik-Rasol (2011), BAP is one of the components on the culture medium that stimulate the production of betacyanins. Some researchers claim that betacyanins can act in the kidnapping of reactive oxygen species (ROS), avoid their accumulation and protecting tissues and cells against damage caused by oxidative stress (Davies et al., 2018).
The betalains have interest because of their antioxidant properties and recently the technical and commercial viability of several in vitro systems for commercial production of these pigments has recently been explored (Georgiev et al., 2008; Pavokovi and Krsnik-Rasol, 2011). The higher staining intensity typical of the betacyanins in the hyperhydric shoots cultivated with BAP indicate the stress condition to which this plant material was exposed. These results allow to broaden the studies on plant stress and its relation with the production of metabolites by plants.
The recalcitrance of the hyperhydric explants corroborates the idea that ROS may be a possible link between oxidative stress and plant regeneration in tissue culture. Hyperhydricity compromises quality and impairs the acclimatization of micropropagated plants, where in commercial micropropagation, about 60% of plants may exhibit hyperhydric symptoms. The correlation of the phenomenon with the accumulation of ROS has been demonstrated by other authors and the present research suggests the existence of an oxidative stress linked to the condition of hyperhydricity (Wu et al., 2009; Tian et al., 2015; Muneer et al., 2018). The excess cytokinin and the reduction of gas exchange in the interior of the vessel to the external environment increased the stress condition, making it difficult to regenerate the tissues (Emara et al., 2018).
Oxidative stress is characterized by the imbalance between ROS generation and the action of the antioxidant system (Mittler, 2002). The evaluation of the activity of antioxidant enzymes demonstrates that the enzyme APX was ahead in the line of defense against oxidative stress. The high APX activity in shoots cultivated at 2.22 BAP and 1.11 μMl l-1 of mT were related to high H2O2 levels in these treatments (Table 3), demonstrating the high affinity of this enzyme for the effective control of H2O2 (Cuypers et al., 2016). On the other hand, the activity of CAT remained unchanged, confirming the information that the increase in H2O2 levels were not enough to cause adjustments in the activity of this enzyme.
Changes in redox metabolism in stressed plants has been evaluated by measuring the content of malondialdehyde (MDA), a lipid peroxidation product that has been used as a biochemical marker for evaluation of stress damage in plants (Wang et al., 2003; Gülen et al., 2008; Rubio-Wilhelmi et al., 2011). The evaluation of MDA allows to determine the level of damage to cell membrane lipids and subcellular membranes. The main consequences of lipid peroxidation in cells are decreased fluidity, reduced membrane selectivity, damage in membrane proteins, inactivation of receptors, enzymes and ion channels. Plants exposed to stress exhibit increased levels of lipid peroxidation due to increased accumulation and generation of ROS (Gill and Tuteja, 2010). In the present work, the results showed that shoots cultivated with 2.22 BAP presented increases of 141 and 67% in MDA contents in relation to treatments with 1.11 BAP and mT, respectively. This result is indicative the change in redox reactions, suggesting a stress condition characterized by increased damage to the lipid membranes.
The results of this work indicate the possibility to use mT in the micropropagation of erect prickly pear in semisolid MS medium (Figure 1 F), with reduction in the occurrence of hyperhydricity (Table 2). The increase in lipid peroxidation and the occurrence of hyperhydricity in the aerial part of O. stricta are indicative of stress that can lead to recalcitrance of the plant material. Thus, studies to improve the morphophysiological quality of micropropagated shoots and increasing the production of seedlings of O. stricta with socioeconomic interest, are important.
CONCLUSIONS
The results obtained in the present research suggest the use of mT in the Opuntia stricta Haw micropropagation. The occurrence of abnormalities in vitro culture compromises the production of seedlings, since the explants may become recalcitrant. The cytokinin type, concentration and nutrient medium consistency may alter regeneration capacity in O. stricta on in vitro culture.














