INTRODUCTION
Bacteria within the Bacillus group are an alternative of biological control against significant agricultural pests. In 1995, the Bacillus thuringiensis (Bt) cry1Ab δ-endotoxin and its genetic material were registered in the U.S. Environmental Protection Agency (EPA) for production of Bt-corn (Zea mays L.) (EPA, 2014).
Bacillus thuringiensis is a spore-forming Gram + bacteria strains that produce crystalline, proteinaceous, parasporal bodies within the sporangia with insecticidal activities. Another species, Bacillus pumilus (Bp) is a Gram +, rod-shaped, spore-forming bacteria, found mainly in soils (Logan and De Vos, 2009). Although, some studies have shown Bp effectivity against certain insects, little is known about the toxicity of B. pumilus against coleopteran. Some strains of Bacillus have plasmids that incorporate cry and cyt genes, whose products have toxic properties to insects.
Plasmids are extrachromosomal DNA molecules of various sizes present in many bacterial species and which replicate independently of chromosomal DNA. Most are circular but there are also double or single strandlines (Snyder and Champness, 2007). Depending on their size (1-250 kbp) they can code from a few proteins to hundreds of them (Snustad and Simmons, 2006). The products of the genes they code generally provide an advantage in terms of adaptation of environments and resistance to toxic substances. Some phenotypic characteristics encoded by genes carried in plasmids provide resistance to antibiotics such as ampicillin, tetracycline and kanamycin, and the production of antibiotics such as bacteriocin active against other bacteria, degradation of complex organic compounds, production of enterotoxins, and the production of restriction and modification enzymes (Li et al., 2016).
B. thuringiensis acquired a long-mobile plasmid which is present in the sporulated cell altogether with millions of crystal proteins with insecticide properties. Most plasmids are not necessary for the survival of the cell in which they reside. However, in certain environmental conditions, a plasmid may be essential for survival. Phenotypes conferred by different plasmids include resistance to antibiotics, production of antibiotics, degradation of complex organic compounds, production of enterotoxins, and the production of restriction and modification enzymes (Sorensen et al., 2005).
The mode of action of the Cry toxins involves an osmotic imbalance of midgut epithelial cells, cell lysis, gut paralysis and death of the insect (Bravo et al., 2007). B. thuringiensis var. tenebrionis has been used as a biocontrol agent for insects in the order Coleoptera (Krieg et al., 1983), and its activity is mainly associated to the Cry 3A δ-endotoxin which is highly active against beetles. Formulations having the Cry 3A δ-endotoxin have been commercially available since the eighties, but some insects have generated resistance (Rahardja and Whalon, 1995). B. pumilus has antifungal properties because it produces fungicides such as bacilomicin D and fengicin (Ramarathnam et al., 2007).
The coffee berry borer (CBB) (Hypothenemus hampei Ferrari, Coleoptera: Scolytidae), is considered one of the most devastating pests of coffee (Coffea spp.) cultivation worldwide. It reduces the weight and quality of the coffee when it is reproduced and fed into the fruit (De la Rosa et al., 2005). The female drill pierces the fruit by the part of the crown reaching the endosperm where it lays its eggs. They hatch at the larval stage, and they cut through the cherry tissue by opening pockets to the main tunnel bordered by the mother. In this stage, it causes more damage to the fruit. The mature female leaves the seed and fertilized by a male brother and moves to another fruit (Damon, 2000). These characteristics of the pest biological cycle attempt against the effectivity of the insecticidal treatments (Méndez-López et al., 2003).
In Puerto Rico, H. hampei is the principal pest of coffee crop, too. It is distributed for all the plantations where occasioning important damages with direct impact in all the production chain. The integrated pest management program in Puerto Rico include several practices to diminish the losses and to protect the fruits for the next production cycle. Among them, one alternative is the biological control with Beauveria bassiana, when the infestation level are over the 7.0% and in more than the 50% of sampled fruits, the female not reach the seed (Gallardo y González, 2015). However, this pest is one of the limiting factor for coffee production and other alternative are needed.
Based on the knowledge gained from the results of different investigations, Bacillus thuringiensis strains would be used to control CBB. In this sense, it has been demonstrated the presence of Cry proteins with potential to CBB biological control in several B. thuringiensis strains (Méndez-López et al., 2003; López-Pazos et al., 2009; Zorzetti et al., 2018). Besides, other proteins and secondary metabolites with insecticidal properties secreted by Bacillus strains has been informed (Brar et al., 2007; Palma et al., 2014).
According to that, the characterization of native Bacillus thuringiensis strains is a valuable tool in order to find new insecticidal toxins. Besides, plasmid identification is useful to understand the toxicology of Bacillus strains with biocontrol properties against insects and it would contribute to manage resistance problems (Khorramnejad et al., 2018). Therefore, this study was conducted to determine the potential of two native Bacillus strains (B. thuringiensis Bt3971 and B. pumilus Bp4185) isolated from coffee ecosystem of Puerto Rico as biocontrol of coffee berry borer.
MATERIALS AND METHODS
Bacterial strains
Native B. thuringiensis Bt3971 and B. pumilus Bp4185 strains isolated from coffee agro-ecosystem of Puerto Rico and conserved in the Agricultural Bacteriology Laboratory collection - Universidad De Puerto Rico Mayaguez were used.
Besides, seven strains of Bacillus thuringiensis subsp. kurstaki, two strains of the subsp. aizawai, and three Escherichia coli integrated to plasmids pKK or pTZ with cry genes were acquired from the Bacillus Genetic Stock Center - BGSC (The Bacillus Genetic Stock Center, Columbus, OH, http://www.bgsc.org/) and used as standards (Table 1).
Table 1. Bacillus strains from Bacillus Genetic Stock Center (http://www.bgsc.org/) used as standards.
Insects
Adults from Hypothenemus hampei were collected from field in the same coffee fruit which showed damage by the berry borer and kept for 24 hours in a wet chamber until the start of the bioassays.
Screening of plasmids
All bacterial strains were cultured on nutrient agar (agar 15 g l-1, meat extract 1 g l-1, peptone 5 g l-1, sodium chloride 5 g l-1, yeast extract 2 g l-1) (Sigma-Aldrich Corporation, St. Louis, MO) supplemented with ampicillin (10 mg m-1). Bacterial cultures were examined for growth after 24 h of incubation at 27 °C. Bacterial strains were inoculated in 22 ml of Luria-Bertani broth (Peptone 10 g l-1, yeast extract 5 g l-1, NaCl 5 g l-1) (Life Technologies, Carlsbad, CA) supplemented with ampicillin (10 mg ml-1), and grew overnight in an orbital shaker at 150 rpm at 27 °C. Under these conditions, absorbance (590 nm) in the range 1.7 to 2.0 was achieved.
Three commercial protocols were used for plasmid extraction: Pure YieldTM Plasmid Miniprep System (Promega Corporation, Madison, WI), Rapid Concert mini preparation kit (Life Technologies Carlsbad, CA), and the Hi-Speed Mini Plasmid Kit (IBI Scientific, Dubuque, IA). The plasmid band size expected by these protocols is between 10 - 100 kb. After extraction, plasmid DNA was quantified in a nanophotometer P360 (VWR International, Radnor, PA) and the ratio of absorbance 260/280 was archived. Plasmid isolation products were loaded in agarose (1%) with 3 µl ethidium bromide and visualized in an UV trans-illuminator. A 1 kb DNA ladder (Life Technologies Carlsbad, CA) was used to compare the size of the bands.
Presence of toxic proteins
To determine the presence of toxic proteins B. thuringiensis Bt3971, B. pumilus Bp4185, subsp. kurstaki (4D-1, 4D-6, 4D-7, 4D-8, 4D-9, 4D-10 and 4D-20), aizawai / pacificus(4J-2 and 4J-5) and the E. coli clones of B. thuringiensis ECE-53, ECE127, and ECE131 were analyzed with SDS-PAGE.
Bacteria grew on orbital shaker overnight at 150 rpm. At the next day, bacteria were centrifuged at 10 000 rpm for 10 min. Cells were lysed using 2 µl of lisozime and 2 µl of DNase I. The cell suspension was incubated for 10 min at 30 °C. The cell suspension was centrifuged at 14 000 rpm for 5 min. Proteins were analyzed using SDS-PAGE. A 50 µl aliquot of proteins was mixed with 20 µl of loading buffer (10% SDS, Glycerol, Mercaptoethanol, 0.1% bromo phenol blue, 0.5 M Tris-HCl, pH 6.8). Acrylamide gel consisted of 30% acrylamide, 2% bis acrylamide, 1M Tris·HCl (pH 8.7), 20% SDS, H2O, TEMED, 10% ammonium persulfate. Samples ran at 75 V for 4 h in a vertical protein gel electrophoresis chamber system (OWL Scientific), with Tris-Glycine-SDS 10X loading buffer (glycine 14.4 g, Tris base 3 g, SDS 1 g, water to complete 1 l). Dye used was 0.025% Coomassie blue and silver stain (silver nitrate 0.2 g, sodium hydroxide 5.25 ml, ammonium hydroxide 350 µl).
Bioassays
The insecticidal activity of B. thuringiensis Bt3971 and B. pumilus Bp4185 was determined in bioassay with H. hampei. The strains were grew in nutrient broth (D(+)-glucose 1 g l-1, peptone 15 g l-1, sodium chloride 6 g l-1 and yeast extract 3 g l-1) (Sigma-Aldrich Corporation, St. Louis, MO). Strains were incubated at 28 °C in tubes with 20 ml for 24 hours in an L.E.D agitator at 150 rpm. Standardized bacterial suspensions were prepared at 0.6, 0.8, and 1.0 of absorbance at 590 nm. Absorbance lectures of 0.6, 0.8 and 1.0 at 590 nm represented approximately 109, 1010 and 1012 CPU ml-1, respectively in both strains. The presence of spores as assessed by microscopic preparations. Aliquots of 20 μl of the bacterial suspensions were placed in a circle inside a 10 x 15 cm Petri dish. Using a thin cell brush, an adult insect was placed in contact with the bacterial suspension for 15 minutes. Inoculated insects were placed (one insect per plate) in glass containers and covered with cheesecloth.
Mortality of insects was evaluated every 24 hours after inoculation until 96 h. A binary variable, which consisted in 0 for dead insects and 1 for live insects was used for evaluation. A randomized complete block design (RCBD) was used. Each block had a control insect (placed in contact with sterilized water) and three bacterial suspensions (0.6, 0.8, and 1.0 A, absorbance units). Each experimental treatment had 11 replicates. Mortality was corrected using the formula of Abbott Corrected mortality (%) = (%MT-%MC)/(100-%MC), where MT = mortality of CBB in treatment, and MC = CBB mortality in the control (Abbott, 1925). The efficacy of treatment was calculated as Efficacy=100*(Ta-Ca)/100-Ca, where Ta is the mortality of the population in the treatment, and Ca is the mortality of the population in the control.
Statistical analysis
Data were analyzed with SAS software version 9.2 (SAS Inc, Cary, NC) using generalized linear mixed models (PROC GLIMMIX). Fisher’s least significant difference (LSD) test at P = 0.05 was used to compare pairs of treatment means. To determine the relationship between mortality and time after inoculation linear regression analysis was conducted using PROC REG.
RESULTS AND DISCUSSION
Plasmids of the size 12 kbp were detected in the strains B. thuringiensis Bt3971, B. pumilus Bp4185 as well as in standard strains B. thuringiensis subsp. aizawai 4J-5, and B. thuringiensis subsp. kurstaki strains 4D-10, 4D-6, 4D-8 and 4D-1 (Figure 1 a).
Using the protocol Promega - Pure YieldTM Plasmid Miniprep System kit, a bigger number of plasmids were detected (Figure 1 c). Strains B. thuringiensis subsp. kurstaki 4D-9, which contains the cry7 gene, and B. thuringiensis subsp. aizawai 4D-20 which contains the genes cry3a, cry3b and cry3c, showed an additional band estimated at 30 kbp (Figure 1 e).
Plasmids of 12 kbp were consistently detected in the strains 4D-1, 4D-7, 4D-8, 4D-9, 4D-10, 4D-20, 4J-2, 4J-5, Bt3971 and B. pumilus Bp4185 without regard of the extraction protocol. E. coli cloned plasmids with cry genes were poorly recovered by these three methods of extraction. Additionally, in E. coli 53 (cry1Ac cloned in pKK223) plasmids were not detected with any of the assayed protocols. The use of three different kits confirmed if the expected plasmid was present or not as kits yielded different bands (Figure 1 a, c, e). Bacillus species have low plasmid copy numbers (approximately 40 per cell) as well as cryptic plasmids (Zhang et al., 2010) which can explain the complexity of plasmid profile in the strains used in this study. Additionally, Lereclus et al. (1982) detected variation in plasmid size (2-80 MDa) and number (1-17) in B. thuringiensis.
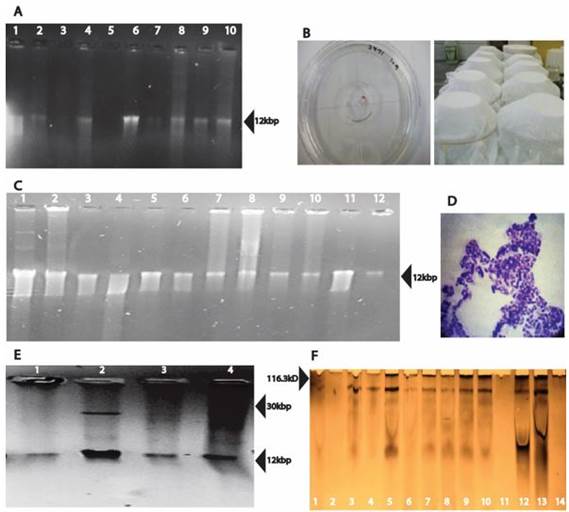
Figure 1. Plasmids from Bacillus strains: (A) Life-Rapid Concert mini preparation kit: 1) Bacillus thuringiensis Bt3971, 2) Bacillus pumilus Bp4185, 3)Bt 4J-2, 4) Bt 4J-5, 5) Bt 4D-20, 6) Bt 4D-10, 7) Bt 4D-9, 8)Bt 4D-6, 9)Bt 4D-8, 10) Bt 4D-1. (B) Bioassays to determine the mortality of coffee berry borer by B. thuringiensis Bt3971 and B. pumilus Bp418. (C) Extraction using the Promega YieldTM Plasmid Miniprep System: 1) Bt 4 D-6, 2) Bt 4D-20, 3) Bt 4D-10, 4) E. coli ECE-127 5), E. coli ECE127, 6)Bacillus thuringiensis Bt3971, 7) Bt 4J-2, 8) Bt 4D-8, 9) Bt 4D-9, 10) Bt 4J-5, 11) Bacillus pumilus Bp4185, 12) E. coli ECE-53. (D) Staining of Bacillus spores. (E) Extraction using the IBI -Hi-Speed Mini Plasmid Kit: 1) Bacillus thuringiensis Bt3971, 2) Bt 4D-9, 3) Bt 4J-2, 4) Bt 4D-20. (F) SDS-PAGE to show the protein profile of: 1) Bacillus thuringiensis Bt3971, 2) Bacillus pumilus Bp4185, 3) Bt 4J-2, 4) Bt 4J-5, 5) Bt 4D-1, 6) Bt 4D-6, 7) Bt 4D-8, 8) Bt 4D-9, 9) Bt 4D-10, 10) Bt 4D-20, 11) Bt 4D-7, 12) E. coli ECE-127, 13) E. coli ECE-53, 14) E.coli ECE-131.
The 12 kbp plasmids detected in the native bacteria B. thuringiensis Bt3971 and B. pumilus Bp4185 were similar in size to the plasmids detected in the Bacillus control group obtained from the BGSC collection (strains of the subsp. kurstaki, and aizawai) that have plasmids and cry genes (Table 1).
In this study, plasmids of 12 kbp were consistently detected in B. thuringiensis Bt3971 and B. pumilus Bp4185. Bacillus thuringiensis produces endospores containing δ-endotoxin proteins toxic to insects and cry genes encode endotoxins. B. thuringiensis has several plasmids whose size varies between 2 to > 200 kbp depending on the strain. The cry genes are found in large (> 40 kbp) conjugative plasmids (which allow gene transfer from one cell to another) (Bravo et al., 1998). Plasmids of two sizes 12 kbp and 30 kbp were detected in this study. A 12 kbp plasmid was detected in the samples of the native strains of B. thuringiensis Bt3971 and B. pumilus Bp4185, and in the subsp. kurstaki and aizawai which have been characterized and identified by having Cry toxins active against coleopteran (Donovan et al., 1992; Ohba et al., 1992; Knowles, 1994). In addition, a 30 kbp plasmid was detected in the Bt subsp. kurstaki, which suggests the presence of more Cry toxins.
In B. thuringiensis, as much as three high-frequency transfer plasmids can be found, and are useful to differentiate between crystal producing (Cry+) and crystal recipient (Cry-) B. thuringiensis- phenotypes. Plasmids or fragments of them from B. thuringiensis have been cloned into commercial vectors (such as pUC, pKK, and pTZ), and transformed into Escherichia coli. This technique allowed locating cry genes, and a deeper understanding of the peptide expression as well as post-transcriptional regulators of endotoxin production (McLean and Whiteley, 1987). In this study, E.coli strain JM 103t containing the cry1Ac gene cloned in a pKK 223 plasmid did not show a band of the expected size for this plasmid (> 40 kbp) indicating a potential loss of the mobile structure. Further studies are necessary to demonstrate the presence of cry genes in the native strain tested. More than 900 toxin genes encoding entomopathogenic proteinaceous toxins, have been identified and characterized in the Bt strains isolated from different regions of the world (Jouzani et al., 2017).
Nevertheless, strains of Bacillus used in this study showed resistance to ampicillin. This characteristic is associated with the RK2 gene, which provides resistance to ampicillin, tetracycline, and kanamycin (Snyder and Champness, 2007). RK2 is the representative archetype of the incompatibility group (Inc P-1) plasmids (Li et al., 2016). In these plasmids, gene clusters associated to antibiotic resistance are mobile and conjugative; therefore, these elements allow bacteria to adapt to environmental stress (Norman et al., 2009).
In the analysis of the presence of toxic proteins by SDS-page, one band of approximately 116 kDa was detected on B. thuringiensis Bt3971 as well as in standard strains 4J-2, 4D-1, 4D-6, 4D-8, 4D-9, 4D-10, 4D-20 and E. coli clones of ECE-53, ECE127 and ECE131 (Figure 1 f). This result denoted the possible Cry proteins presence in the strain.
The δ-endotoxins are made up of two types of proteins, the Cry and Cyt proteins, which have been identified for their insecticidal activity, and δ-endotoxins have molecular masses between 27 to 140 kDa (Ben-Dov et al., 2001; Pardo-López et al., 2013; Jouzani et al., 2017). In this study, it was only possible to identify the presence of proteins in that range for B. thuringiensis Bt3971 bacterium, with 116 kDa. A band of similar weight was detected in Bacillus belonging to the subsp. kurstaki, aizawai/pacificus, as well as the E. coli clones of B. thuringiensis which are characterized by possessing cry genes with proteins toxic to beetles. B. thuringiensis is characterized by the formation of parasporal crystals protein called δ-endotoxins, which are formed during sporulation and are toxic against insects (Logan and De Vos, 2009).
B. thuringiensis Bt3971 and B. pumilus Bp4185 produced a mortality of 18% and 9% at 48 h after inoculation at A590=0.6 (ca. 109 CPU ml-1), respectively (Table 2). At the same time with A590=1.0 (ca. 1012 CPU ml-1) mortality was 36% in both strains. Therefore, the effect of concentration was significant on mortality for both strains at 48 h. At A590=0.6 and A590=0.8, higher mortality rates were obtained in B. thuringiensis Bt3971 than in B. pumilus Bp4185 after 96 hours of inoculation.
Table 2. Susceptibility of Hypothenemus hampei to Bacillus thuringiensis Bp3971 and Bacillus pumilus Bp4185.
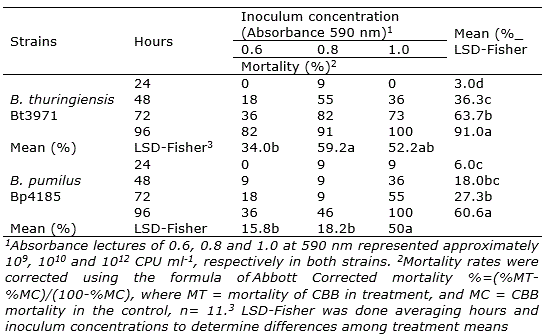
In B. thuringiensis Bt3971, mortality rates at the concentrations of A590=0.8 and A590=1.0 were significantly higher than at A590=0.6 (LSD - Fisher; α = 0.05). In B. pumilus Bp4185, mortality at A590=1.0 was significantly higher (LSD - Fisher; α = 0.05) than A590=0.8 and A590=0.6, averaged over time.
Positive linear effects (p<0.001) were detected in the relationship mortality and inoculation time; therefore, the higher the time after inoculation, the higher the mortality rates in both strains. The shortest time at which approximately 50% of the insects (six insects) were killed with B. thuringiensis Bt3971 was 48 h at A590=0.8, and with B. pumilus Bp4185 was 72 hours at A590=1.0. Mortality rates showed that B. pumilus Bp4185 and B. thuringiensis Bt3971 were effective in controlling H. hampei. B. thuringiensis reached mortality rates using smaller inoculum concentration than B. pumilus.
B. thuringiensis Bt3971 showed high toxicity against the coffee berry borer, expressed as high mortality rates as soon as 48 h after inoculation. Results were similar to those observed by Méndez-López et al. (2003) and De la Rosa et al. (2005) with different strains of B. thuringiensis for the control of the coffee borer in larval and adult stages using Bt-spore-crystal complexes. In the case of Méndez-López et al. (2003) these authors determined that Bt subsp. israelensis was highly toxic to the coffee berry borer during its larval stage. They used spore-crystal complexes of Bt strains at different concentrations (1000-10.1 ng cm²) in an artificial diet through bioassays using larvae of the first instar of the drill. On the other hands, De la Rosa et al. (2005) performed bioassays using different growth stages of the coffee berry borer such as eggs, larvae and adult females. They analyzed the spore-crystal complexes of 61 strains of Bacillus thuringiensis mixed in an artificial diet at 1.0, 10.0 and 100 μg g-1 of spore-crystal complexes. Bt strains showed different degrees of toxicity in larvae of H. hampei, with mortality ranges from 8.3 to 83%. Similarly, Zorzetti et al. (2018) informed that 11 B. thuringiensis strains (32.3%) caused mortality to H. hampei larvae that exceeded 96%.
Bacillus pumilus forms ellipsoidal spores located in the central part of the cell, whose natural habitat is mostly the soil (Molina et al., 2010). In the present study, B. pumilus Bp4185 strain showed 12 kbp plasmid. The results demonstrated that not only the Cry proteins would have toxic effect on H. hampei applied in the diet (Méndez-López et al., 2003; López-Pazos et al., 2009) and other bacterial strains has potential to control this pest. In this sense, a strain of Bacillus pumilus has been patented for controlling corn rootworm, nematode, and armyworm infestations (Heins et al., 1999) and different authors reports the use of this species for controlling other insects. For instance, Molina et al. (2010) using larvae of Ceratitis capitata, in bioassays with B. pumilus showed mortality between 68 to 94% after 15 days of inoculation in larvae of the first instar. Similarly, Yaman and Aslan (2010) in Dendroctonus micans (Coleoptera: Scolytinae) showed that B. pumilus caused 69% and 40% mortality in larvae and adults, respectively. B. pumilus Bp4185 showed a mortality of 54.5 to 100% between 72 h and 96 h after inoculation, with higher mortality and shorter inoculation time than that obtained by the authors mentioned above.
CONCLUSIONS
The Puerto Rican native strains Bacillus thuringiensis Bt3971 and Bacillus pumilus Bp4185 have the presence of plasmid (12 kbp). Besides, the presence of a protein with a molecular weight band of 116 kda in B. thuringiensis Bt3971 denote the possible presence of Cry proteins. This is the first study demonstrated the insecticidal activity of B. thuringiensis Bt3971 and B. pumilus Bp4185 on H. hampei. According to that both strains have potential for coffee berry borer biocontrol.














