INTRODUCTION
The quinoa (Chenopodium quinoa Willd) is a pseudo cereal rich in proteins and minerals grown for thousands of years and serving as food for populations from various regions of the world (Tapia, 2014) due to its characteristic of adaptation in several agroecosystems (Jarvis et al., 2017). Because of its great nutritional value, quinoa has been gaining productive prominence in several regions and can even be grown in irregular soils (Jarvis et al., 2017), whereas for other crops, this condition becomes impossible or unproductive.
When considering these and other characteristics, the quinoa culture is still little explored if compared to species of great commercial appeal (Massawe et al., 2016). However, since the 1980s, its consumption has strongly increased, especially by consumers (Silva et al., 2013), based on the philosophy of organic production. In relation with food security and the environment, scientific works aimed to proposing alternatives for phytosanitary control has become more frequent, for example, using plant extracts, instead of chemical products (Luz et al., 2007).
The seed treatment is essential to guarantee productivity. The fungal control over the seed surface contributes to the reduction of microflora and allows increasing the germination percentage, both in laboratory conditions and field (Medeiros et al., 2015).
Species of medicinal plants can show activities through their secondary compounds and these can be easily extracted (Venturoso, 2009). It would be used in the form of plant extract for the quinoa seed treatment, if proving to be efficient. For instance, authors like Venturoso et al. (2011) concluded that there was a fungistatic activity over the in vitro growth of Phomopsis spp., Colletotrichum spp., Aspergillus spp., Penicillium sp., Fusarium solani and Cercospora kikuchii when using aqueous extracts of clove (Syzygium aromaticum L.).
S. aromaticum L. (family Myrtaceace) is native to Indonesia and part of the dry floral bud has been widely used for centuries in cooking and medicine as an anesthetic and antiseptic on account of substances found like eugenol and used for many purposes (Batiha et al., 2020).
Mazzafera (2003) demonstrated that seeds treated with plant extracts of S. aromaticum strongly inhibited germination, in which eugenol actively took part. However, with the use of the 10% aqueous extract of this species, Lidório et al. (2020) informed a sanitary control index higher than 28% of the total fungal infested seeds in quinoa. In this regards, is important to know the cytogenotoxicity potential of S. aromaticum extract on seeds.
The verification of the degree of toxicity of this species can be performed using the Allium cepa test, in which it may be possible to establish the mitotic index in treatment with plant extracts in genotoxicity studies. The test has been widely used in several fields. Its results can possibly characterize them as bioindicators of genotoxicity by counting the onion root cells damage after exposed to the evaluated substances. This is essential in order to verify what the toxic effects are. It can be observed by cellular inhibition, disruption in metaphase, induction of chromosomal aberrations, among others. Besides, is possible to perceive the probable important changes in the plant chromosomes (Tedesco and Laughinghouse, 2012)).
In the search of seed treatment with low environmental impact, this study aimed to evaluate the cytogenotoxicity potential of the aqueous extracts of S. aromaticum, an alternative to Chenopodium quinoa Willd seed treatment.
MATERIAL AND METHODS
Plant material
The lot of quinoa seeds came from an experimental cultivation area at the Santa Maria Federal University (UFSM), in 2018. The seeds of lot Q-1303 were harvested, cleaned and stored for five months in a cold chamber (15 °C and 40% RH), with initial humidity of 12.2%. S. aromaticum (clove) flower buds were obtained from the local organic trade in the city of Santa Maria/ BR.
Experiment design
The experiment was conducted in 2019, at the Didactic and Seed Research Laboratory of the Federal University of Santa Maria, RS, in an open randomized design. It was constituted with five treatments: clove extract at 2%, 4%, 6% and 8% (w/v) and control without extract, with four repetitions. One hundred (100) seeds composed each experimental unit.
The aqueous extracts were produced from S. aromaticum flower buds, according to Mazaro et al. (2008) with adaptations. The flower buds were processed in a shredder with 100 ml of distilled water at room temperature to obtain extract at 2%, 4%, 6% and 8% (w/v). The liquid of each extract was stored in the absence of light and at room temperature for 24 h. In the aftermath, the extracts were filtered, separately, on Watman paper No. 1, packaged and identified. After obtaining the extracts, the quinoa seeds were submerged for ten minutes in each treatment and the control (only distilled water), at room temperature. The seeds response were evaluated by the following tests.
Germination test
The germination test was performed jointly with the test of the first germination count, germination speed index and the emergence. It was employed four repetitions of 100 seeds accommodated in Gerbox® boxes with three sheets of filter paper moistened with distilled water at 2.5 times of its weight. The seeds were arranged in a germination chamber of the type BOD (Box Organism Development), 20 °C in six days with a photoperiod of 16 h (Brazil, 2009a).
Daily evaluations were carried out to determine the germination speed index, according to the methodology proposed by Maguire (1962) and alongside, the analysis of germination and vigor were performed on the fourth and sixth day after sowing (DAS). Afterward, the normal seedlings were counted using the parameters of 1.5 cm of aerial part and with the developed root system, characterizing them as normal plants. The results expressing the evaluations in percentages of normal seedlings, abnormal seedlings, damaged and infected and total dead seeds (Brazil, 2009a).
The emergence evaluation was performed 14 days after sowing (DAS) and the emergence speed index was determined by daily evaluations according to the methodology suggested by Maguire (1962).
Seedling length test
For the seedling length test, four replicates of 100 seeds were used. The seeds sown in Gerbox® and held under the same germination conditions described above. The seedling length (mm) above ground and the root length (mm) were randomly assessed on ten normal seedlings by replication, each day after sowing (DAS) (Nakagawa, 1999).
Sanity test
In the sanity test, four replicates of 100 seeds were used, incubated in paper substrate (Blotter test), following the same methodology mentioned above. Then, the germination was inhibited by the freezing method for 24 h and after that, the seeds returned to the BOD, remaining there for five days, at 20 ± 2 °C and with a photoperiod of 12 h. The result of the count of infected seeds was expressed as a percentage of infested seeds and the genera of infested pathogens were assessed individually evaluated with the aid of a magnifying glass (microscope stereotype) and traditional manual (Brazil, 2009b).
The data were transformed into arc sine √(x/100) and the analysis of data variance was performed and the averages were compared using the Tukey test (p<0.05) using the statistical program R Core Team.
Allium cepa test for possible chromosomal changes
The Allium cepa test was held at the Plant Cytogenetics and Genotoxicity Laboratory at UFSM according to the general description of Tedesco and Laughinghouse, 2012). The cells in the meristematic region of the plant bioindicator were analyzed in order to observe possible chromosomal changes during the cell division. To evaluate the genotoxicity of the aqueous extracts of S. aromaticum, a completely randomized design was used, with six treatments composed of four concentration of flower buds extract, positive control (glyphosate 1%) and negative control (distilled water at room temperature).
The S. aromaticum extracts (2-8%, w/v) were obtained as described above. Subsequently, they were organized, with four replicates each, containing four bulbs of Allium cepa L. for treatment.
The bulbs arose from a population previously tested to obtain a plant material with minimal changes in the natural environment and, at first, they had their old roots shaved and then placed for rooting in distilled water for three days. After rooting, with the exception of the negative control treatment that remained the same, distilled water was replaced by the treatments mentioned above. Then, the roots remaining for another 24 h and, after this period, it were collected, fixed in ethanol: acetic acid (3:1) for 24 h and, subsequently, stored in 70% ethanol under refrigeration, until the analysis of cell division (Souza et al., 2010).
For the analysis and cell counting, two laminas per bulb were made and 500 cells were counted per lamina.
The laminas were prepared using the methodology adapted from Guerra and Souza (2002), where the roots were hydrolyzed in 1N HCl for 5 minutes, then washed in distilled water at room temperature and had the meristematic region removed and the cells were stained with a drop of 2% acetic orcein. The meristematic region was crushed and a glass cover slip was applied on this material. With the aid of optical microscopy in 40X magnification, 500 cells per lamina were analyzed, taking into account the phases of cell division and the chromosomal changes found. Subsequently, the mitotic index (MI) of the treatments was calculated, based on the percentage of cells in division and the genotoxic potential, related to the amount of cellular changes using the methodology adapted from Guerra and Souza (2002).
The data were normalized by the Box-cox test and their normality was tested by the Shapiro-Wilk test and the analysis of variance was performed, comparing the averages through the Tukey test (p<0.05) using the statistical program R Core Team.
RESULTS AND DISCUSSION
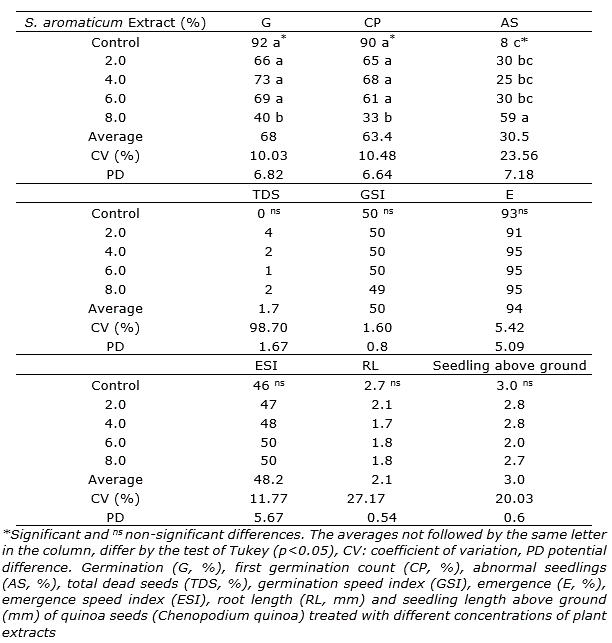
The seed of quinoa lot showed 92% germination. The aqueous extracts of S. aromaticum in concentration from 2 to 6% not affected the germination and vigor in quinoa seeds. It was noticed that with the increase of the concentration of the flower buds extracts to 8%, the germination percentage and vigor were reduced to 40% and 33%, respectively. Besides, the rest of variables (total dead seeds, germination speed index, emergence, emergence speed index, root length and seedling length above ground) not showed significantly differences in comparison with the control treatment (Table 1). Therefore, the results with 8% indicated a negative effects and the toxic potential of the S. aromaticum extract, when it used in higher concentrations.
Similar results were found by Mazzafera (2003)that applied different concentrations of S. aromaticum as a treatment for seeds of cape periwinkle (Catharanthus roseus L.), wheat (Triticum spp.), radish (Raphanus sativus L.), ryegrass (Lolium multiflorum Lam.), maize (Zea mays L.), arnica (Arnica lanceolata Nutt.), rumex (Rumex obtusifolius L.) and mustard (Sinapis alba L.). The author describes that the great majority of the concentrations inhibited the seeds germination. In this sense, Iganci et al. (2006) concluding that substances with allelopathic effects contribute to the inhibition of seed germination, negatively affecting the growth and activation of enzymes for cell division.
According to Rabêlo (2010), eugenol is the main compound found in the S. aromaticum species, which presents toxic characteristics to living organisms when in high concentrations. In agreement with the author, it was noticed that the increase in the percentage of the aqueous extracts of S. aromaticum as a treatment for quinoa seeds caused toxicity to them (Table 1). Especially, it was observed an increase in the number of abnormal seedlings in the highest tested concentration (8%), with 59%, in comparison with the control treatment during the tests.
Table 1. Effects of different concentrations of Syzygium aromaticum extracts in the treatment of quinoa seeds.
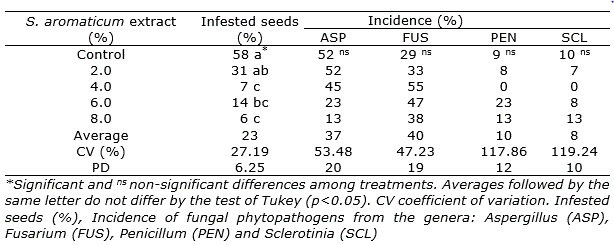
The fungal pathogens found in the quinoa seeds were Aspergillus, Fusarium, Sclerotinia and Penicillum, with an incidence of 52%, 29%, 10% and 9%, respectively, in the control treatment (Table 2). The use of the aqueous extracts of S. aromaticum from 4 to 8% proved to be positive for reducing the incidence of infested quinoa seed and they would be an alternative to seeds treatment. The results are in accordance with previous reports of antifungal effects of S. aromaticum extract. For instance, Avasthi et al. (2010) indicated in vitro antifungal activity of aqueous extract of flower buds on Aspergillus niger, a causative agent of different disease. Rana et al. (2011) informed antifungal efficacy of clove oil towards Trichophyton rubrum, Microsporum gypseum, Fusarium moniliforme, F. oxysporum, Mucor sp., M. gypseum and Aspergillus sp. In the same way, Lidorio et al. (2020) that demonstrated a significant reduction in the infestation of quinoa seeds by fungi with the use of aqueous extracts of this species.
Table 2. Effects of different concentrations of Syzygium aromaticum aqueous extracts on the control of fungal pathogens in Chenopodium quinoa seeds.
The analysis for the concentrations of aqueous extract of S. aromaticum resulted in different levels of antiproliferative actions in all treatments, when compared with the control (Table 3).
The use of different concentrations of the aqueous extract of S. aromaticum in roots of A. cepa showed a significant difference among the mitotic index averages of treatments and the negative control (water). The results showed that these concentrations have greater inhibitory activity of cell divisions, similar with the positive treatment (Glyphosate, 3.75%). According to that, the aqueous extracts of S. aromaticum can be characterized with antiproliferative action, inhibiting cell division and in consequence cytotoxic effect.
The results could related with the slightly levels of toxicity observed in the germination and vigor analysis of the seeds, more evident in the higher concentration tested, as described above. Mazzafera (2003) concluded that the compounds present in plant extracts of medicinal species can have mutagenic effects and decrease cell proliferation when is in direct contact with another species. As well as how, secondary metabolites that are present in the aqueous extracts of S. aromaticum, among them, eugenol, causing representative allelopathic effects (Rabêlo, 2010; Batiha et al., 2020).
Table 3. Mitotic index of the treatments used as a way to evaluate the cytotoxic effects of Syzygium aromaticum extracts in roots of Allium cepa.
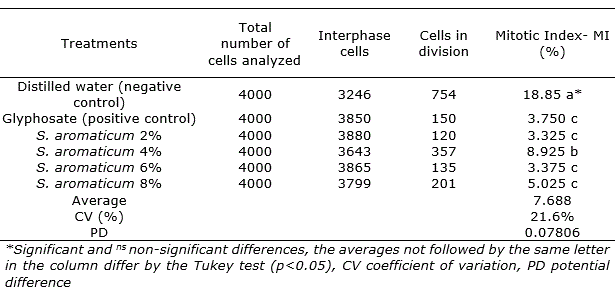
The evaluation of MI in A. cepa test is an indicator of direct action of toxic substances on DNA (Tedesco and Laughinghouse, 2012); Bonciu et al., 2018). Conversely, during the observations, no cells with chromosomal alterations were found and it indicated that S. aromaticum did not causes genotoxic effect. Reports on the cytotoxic and genotoxic effects of clove in seed treatment are scarce, although the flower buds and its extracts are recognized as safe for other uses like food supplement and medical applications (Vijayasteltar et al., 2016; Batiha et al., 2020).
The aqueous extracts of S. aromaticum exhibited outcomes in the control of fungal quinoa seed pathogens (Table 2), indicating a possible gain in the use of this species as unconventional seeds treatment. According to the results, 4% aqueous extract of S. aromaticum could be used in the treatment of quinoa seeds. However, approximately 20.0% more of seeds should be added, in order to achieve germination similar to control treatment (Table 1). Further studies are required to characterize the effects of S. aromaticum extract on quinoa plant development.
CONCLUSIONS
The treatment of quinoa seeds with aqueous extract of S. aromaticum provides benefits in terms of antifungal activity without negative effects over germination and vigor except when treated with elevate concentrations (8%). The extract cause cytotoxic effect evaluated by mitotic index but not genotoxic effect. Used at 4% (w/v) it could be an alternative for quinoa seed treatment.














