Introduction
In a previous publication we described the life and career of Frederick Crace-Calvert (1819-1873) (Figure 1) and his work in biology and biochemistry (Wisniak, 2021). Here we describe his contribution to inorganic chemistry, in particular the chemical properties of lead, the synthesis and properties of a large number of new lead derivatives, improvements in the manufacture of iron and steel, the synthesis and properties of new alloys and amalgams, and the therapeutic properties of picric acid.
Lead derivatives
The first paper on the subject was a short note regarding the behavior of the hydrate of lead protoxide (PbO.H2O) in contact with a boiling solution of concentrated NaOH (35 to 42%) (Crace-Calvert, 1843). After cooling, the resulting liquor deposited a pink precipitate of lead oxide, crystallizing as regular cubes. Upon heating to about 400 oC, this oxide swelled, turned black, and released about 1/1000 parts of water. Heated to cerise red (about 1000 oC), it turned sulfur yellow, without changing its crystal form. The oxide was slightly soluble in all the acids, particularly in dilute or concentrated nitric acid. In powder state it was orange yellow like litharge. The dehydrated material had a composition (per weight) of 92.83% lead and 7.17% oxygen. Addition of the original hydroxide to melted NaOH turned it instantly into an amorphous isomeric red substance. When powdered, this new oxide turned yellow red and was very soluble in acids. Crace-Calvert also found that the solution of the hydrate of lead protoxide into saturated KOH generated a third oxide, which seemed to be identical to the one reported by Eilhert Mitscherlich (1764-1863) (Mitscherlich, 1818-1819; Crace-Calvert, 1843).
Crace-Calvert reported that a sheet of lead behaved in a completely manner when submerged in distilled water or in common (saline) water: In the first case the sheet became covered with a white layer, probably of lead carbonate, while the water become milky after a few minutes. In the second case, no reaction was observed in the metal or in the solid and gaseous substances contained in it, even after a long time of contact, while the water remained transparent (Crace-Calvert, 1845). An extended experiment consisted in putting pieces of lead in stoppered bottles containing recently distilled water, aerated distilled water, Seine river water, and water from several wells and springs in Paris. After a few days all these liquids were treated with hydrogen sulfide. The bottle containing distilled water and aerated distilled water showed an intense coloration of lead sulfide, while the river and spring waters showed a very weak shade of this chemical. To avoid possible errors Crace-Calvert employed distilled free of HCl collected from a continuous operating distillation apparatus. Crace-Calvert explained the low concentration of lead in common waters because its salts had been probably eliminated by the soluble sulfates, carbonates, and chlorides usually present in this kind of waters (Crace-Calvert, 1845, 1861c).
In a following paper Crace-Calvert referred again to the three lead oxides he had previously obtained. He now stated his belief that the red color of the third oxide, which was amorphous and very similar to minium, was the result of a molecular change caused by heat (Crace-Calvert used the term caloric) and not to the presence of very small amounts of lead peroxide, free or combined (Crace-Calvert, 1846). He now reacted the hydrate of lead protoxide with ammonia under similar conditions as with alkalis and obtained crystalline yellow-green products, which were found to be combinations of the two reagents, in the ratios 3PbO.NH4O and 8PbO.NH4O. These compounds should be considered to be ammonium plumbates containing one equivalent of water, and according to Crace-Calvert, they seemed to be the first-ever prepared compounds of ammonia with a metallic oxide. This finding suggested the possibility of combining ammonia with all the metals (Crace-Calvert, 1846). Crace-Calvert also reported that he had reacted ammonia with a concentrated solution of lead nitrate and obtained a series of salts, mostly crystalline, which were of interest because they contained ammonia combined in a particular state. Upon slightly heating, these salts turned yellow while releasing water and ammonia. On cooling, they became white and heated again they released radiant vapors and turned into beautiful yellow massicots, hardly fusible. Crace-Calvert believed that these new salts were lead nitrates containing different elements of hydration or crystallization, such as 2(NO5,6PbO) +3HO, 2(NO5,5PbONH3) +3HO, 2(NO5,4PbONH3) +3HO, and 2(NO5,3PbONH3) +3HO (Crace-Calvert is using the old nomenclature where HO is the formula of water). He named these compounds hydrated nitrates of ammoniated lead because one equivalent of ammonia was gradually substituted by one equivalent of lead oxide, in the same manner that in organic chemistry chlorine replaced hydrogen. He reported that he had also synthesized salts where the ammonia had not reacted with the lead oxide contained in the nitrate but with its water of crystallization, for example, tribasic nitrate of hydrated lead, 2(NO5,3PbO).3HO and tribasic nitrate of hydroammonia lead, 2(NO5,3PbO).2HONHO (Crace-Calvert, 1846).
The last publication described in much more detail the preparation and properties of the different lead oxides (three crystalline, one olive, one red, and one pink, and the fourth amorphous and of a fine red), the action of ammonia on the hydrate of lead protoxide, the action of ammonia on the nitrates of lead at different temperatures, the various ammonia nitrates (hydrated and anhydrous), and the action of ammonia on the boiling solutions of these salts, with the following conclusions (Crace-Calvert, 1851): (1) the color of the red oxide had been improperly attributed to the presence of a small amount of PbO2. In practice, the color was due to a molecular change that massicot experimented when heated in an oven; (2) Crace-Calvert was able to combine one part of lead oxide with one of ammonia and synthesize a plumbate of ammonium oxide or ammonia plumbate combined with one equivalent of water; (3) he had also succeeded in synthesizing a variety of salts in which one equivalent of lead oxide was gradually substituted by one of ammonia. In his words "the ammonia replaced the oxide of lead without changing the harmony that existed in the molecular arrangement of the composing elements of the salt"; (4) hydrated trinitrate and hexabasic nitrates were prepared in which the same number of ammonia equivalents replaced one or two equivalents of water of crystallization. The molecular arrangement of these insoluble salts was shown to depend on the concentration of the solutions and the operating procedure; (5) a variety of new salts were prepared that held tenaciously a small amount of nitric acid, and (6) addition of KOH to lead nitrate resulted in the formation of several new lead sub-nitrates (Crace-Calvert, 1851). Some new details were added, for example, acetic acid dissolved rapidly the white lead oxide and very slowly the yellow one. Hence it could be used to separate the two oxides. Treatment of white lead oxide with melted NaOH produced instantly the red amorphous variety; temperature was the only factor influencing this color change. The red crystalline oxide was prepared by adding boiling KOH to the hydrate of lead oxide. The olive oxide was made mixing a warm solution of lead nitrate (of specific gravity 1.114 at 15.6 oC) with a warm solution of concentrated KOH (of specific gravity 1.162 at 15.6 oC) and then boiling the resulting solution of lead sub-nitrate for one hour. This oxide was completely soluble in acids (Crace-Calvert, 1851).
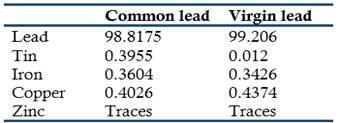
According to Crace-Calvert and Richard Johnson many scientists believed that the purer a metal was the least it was attacked by acids, an opinion that had led the producers of metals to try to sell metals as pure as possible. This tendency was particularly typical among the manufacturers of lead destined to the making of sulfuric acid by the method of lead chambers. This opinion led Crace-Calvert and Johnson to test the truth of this assumption for the different commercial leads available (Crace-Calvert & Johnson, 1863). For this purpose, they carried a series of experiments to study the effect on lead of sulfuric of different concentrations and purity, different volumes, and at various temperature levels and times of contact. The lead tested was of two degrees of purity: common sheet lead and virgin or Derbyshire lead, having the following composition:
In addition, they prepared and tested a sample of chemical pure lead. The tests were conducted on lead plates of one m2 in contact with 16 liters of sulfuric acid chemically pure and of different densities, and during 10 days at room temperatures (18o to 20 oC). Each test was repeated 3 or 4 times. The amounts of lead dissolved as lead sulfate, were the following:
These results clearly indicated that that the actual corrosion was exactly the opposite as claimed by common opinion: the higher the purity of the metal, the higher the amount of corrosion by sulfuric acid. Additional experiments conducted at 40o to 50 oC with sulfuric acid of relative density 1.746 yielded the same conclusion.
Carbon
Crace-Calvert wrote that it was well known that the presence of sulfur in iron rendered it brittle when heated red. This sulfur rarely originated from the mineral; it usually came from the fuel employed. His efforts to find a method for decomposing these sulfides from all possible sources had led him to discover that sodium chloride, applied in a certain manner and appropriate proportions, provided the required solution (Crace-Calvert, 1852). His results indicated that hot iron bisulfide decomposed into the monosulfide and the latter, in the presence of sodium chloride, decomposed into iron chloride and other compounds. This chloride, at high temperatures and in the presence of steam, split into iron oxide and HCl. In the blast furnace the sodium and chlorine become part of the slag and not of the iron. Tests made in three blast furnaces had shown the advantages of this process, as seen in the samples that Michel Eugène Chevreul (1786-1889) presented to the Académie des Science.
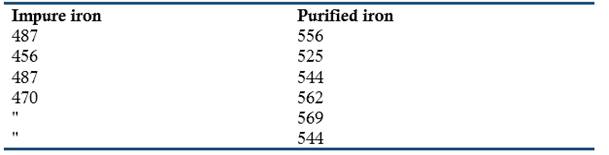
Examination of the improved iron showed the disappearance of the crystals that decreased its tenacity and the appearance of long fibers that improved this property significantly. Crace-Calvert reported that he had not yet determined the change in tenacity that occurred in malleable iron produced by his process, but he had measured the relative resistance of cast iron produced by the standard and the improved procedure. Application of a gradual pressure in the center of two bars had given the following results:
Crace-Calvert wrote that the bar of impure iron contained 6/1000 parts of sulfur and the purified one 1/1000 parts. He added several examples of the large-scale application of his method in England and in France; for example, in England it had been used in the manufacture of the coke employed in the production of the iron for a very large train line. The results indicated that this coke was completely free of sulfur (Crace-Calvert, 1852, 1855).
A short note published in 1871 described an easy procedure to determine the amount of sulfur contained in carbon and in coke (Crace-Calvert, 1871). Crace-Calvert wrote that it was known that sulfur was found in the substances partly as part of sulfuric acid combined with lime, and partly as sulfur combined with iron (sulfide). The latter compound was important because it decreased the value of commercial iron. According to Crace-Calvert the first derivative could be simply determined by boiling pulverized coal or coke with sodium carbonate, which decomposed calcium sulfate or sulfide and transferred the sulfur to the liquid phase (Crace-Calvert, 1871).
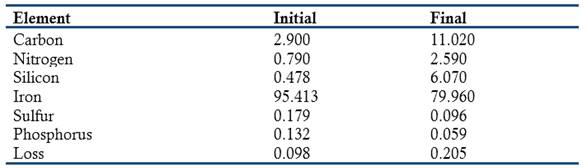
In another publication, Crace-Calvert wrote that he had found that the solution of the cast iron in HCl always left a carbureted residue in an amount that varied with the concentration of the acid. This led him to study the possible action of diluted acid upon this iron (Crace-Calvert, 1861a, 1862). In his first experiments he used samples of cast iron shaped as cubes of one cm dimension. These cubes were put in corked bottles with eighty times their weight of weak solutions of HCl, sulfuric, nitric, and acetic, oxalic, tartaric, and gallic acids. After three months he noted that spite of the dissolution, the cubes did not seem to change their apparent shape and size. After several months, the blade of a penknife was able to penetrate 3 to 4 mm inside the cube. After two years of being under the action of a dilute acid (renewed after some time) the cast iron cubes had not lost their shape and had transformed completely into a graphite-like substance that could be penetrated from side to side. During this period, the weight of the cube had decreased from 15.324 g to 3.489 g; in other words, it had lost 77.13% of its iron, carbon, sulfur, phosphorus, and silicon. An interesting finding was that acetic acid was the most effective material, it seemed to act in a continuous manner and there was no need to renew it. Crace-Calvert analyzed the cast iron before and after the acid treatment and obtained the following results:
According to Crace-Calvert, the above results indicated: (a) the considerable amount of nitrogen present in the graphite-like substance; (2) all acids led to the release of silane during their action; (3) the amount of carbon present in the cast iron was larger than that given in the table because an oily substance was always released during the acid attack; (4) the graphite-like substance always contained carbon and iron in the ratio 4C: 6Fe; and (5) exposed to air, the graphite-like substance heated rapidly due to the oxidation of its iron (Crace-Calvert, 1861a, 1862).
Crace-Calvert repeated the above experiments using steel instead of cast iron (Crace-Calvert, 1861b). This time he cut two strips of steel from the same plate; tempered one of them and then submerged both in a solution of diluted acid. He observed that the tempered strip dissolved in the acid leaving a deposit that looked like soot while the non-tempered strip kept its shape and thickness, and transformed into grey graphite, containing iron, carbon, and a little of nitrogen.
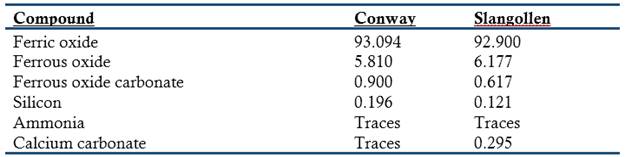
A curious research originated from question put to Crace-Calvert by Charles Fox, the engineer of the Palais de Crystal for the exposition of 1851: What is the composition of rust (Crace-Calvert, 1870)? In order to answer the question, Crace-Calvert analyzed rust collected from places far from manufacturing centers. The results surprised him; he found that the composition of rust was far more complicated than the one given in chemistry books. Typical analysis from two sites provided the following results:
These results led Crace-Calvert to investigate the atmospheric components that were responsible for causing the oxidation of iron (water vapor, oxygen, or CO2). In order to answer the question Crace-Calvert put strips of well-scrapped iron inside a vessel containing pure mercury, inverted it over a tank of the same metal, and added to the tubes pure oxygen, wet oxygen, ammonia, and the same gases mixed with a small amount of CO2. The bottom of the strips was covered with gutta-percha to avoid contact with the mercury. After a few days he observed that the oxidation had taken place irregularly. No reaction had taken place on the strips in contact with dry oxygen, dry CO2, and a mixture of dry oxygen and dry ammonia. All the other strips were attacked to a certain degree. The faster attack occurred on the strips in contact with a mixture of wet oxygen and CO2. According to Crace-Calvert the results showed clearly that the main atmospheric factor causing rust was CO2 and not oxygen or water vapor (Crace-Calvert, 1870).
Crace-Calvert wrote that Nicolas Théodore de Saussure (1767-1845) discovered that carbon had the property of condensing several times its volume of different gases, and among others, ammonia and HCl in the proportion of 80 to 90 times its volume (Saussure, 1812; Crace-Calvert, 1867c). This adsorption ability was considered to be a physical property until John Stenhouse (1809-1880) showed that under the influence of carbon oxygen was able to combine with several of the products released during the phenomenon of putrefaction. This oxidation was so fast and complete that the putrefying animal, kept in carbon, could continue in this state without releasing any obnoxious odor (Stenhouse, 1854-1858). These results led Crace-Calvert to study the oxidation power of oxygen in this situation and the possibility of extending it to the oxidation of mineral and organic substances. His experiments were conducted using carbon cubes originating from the calcination of box tree. These cubes were first boiled with a diluted solution of HCl and then with distilled water. These operations were conducted in order to free the carbon from mineral substances, particularly alkaline carbonates that could affect the results (normally an acid material produced in strong oxidations). After drying, the cubes were heated red and, while hot, introduced in a cylinder standing over mercury and containing a known volume of oxygen. The gas to be oxidized was then added and the whole left until the column mercury ceased to elevate, signaling the end of the absorption process. The cubes of carbon were also examined to assure they did not contain CO2 (Crace-Calvert, 1867c).
Crace-Calvert tested the action of condensed oxygen on three kinds of substances, mineral (i.e. moist SO2, hydrogen sulfide, ammonia, and phosphine); alcohols (i.e. methanol, ethanol, and amyl alcohol), and hydrocarbons (i.e. ethylene, propylene, and amylene) and reported the following results: (1) SO2 was oxidized to SO3, hydrogen sulfide into sulfuric acid and water, and phosphine into phosphoric acid and water. All intents to oxidize ammonia into nitric acid failed; (2) methanol was oxidized to a low extent into formic acid, ethanol was significantly oxidized into acetic acid, and amyl alcohol into valeric acid; (3) ethylene and propylene were oxidized into water and CO2; amylene was converted into water, CO2, and a third component, which seemed to be amyl valerate, Crace-Calvert believed that the oxygen condensed in the carbon induced the condensation of the oxidizable material by simple molecular attraction. The mutual affinity of the two liquids would then lead to their reaction (Crace-Calvert, 1867c).
Metals, alloys, and amalgams
Crace-Calvert and Johnson wrote that alloys and amalgams, in spite of their scientific and practical applications, had been little studied (Crace-Calvert & Johnson, 1855). Their manufacture was based on arbitrary recipes not based on the law of chemical combinations. Since almost all chemical compounds resulted from the chemical combination of their components, the same argument should be applicable to metals when contacted under conditions favorable to their mutual combination. If no definite compound was produced it was probably caused by the excess of one or more of the metals that entered in the composition of the alloy and the probable solidification of this excess. This was a known fact for the alloys of bismuth and sulfur in a crucible: upon cooling of the mixture, crystals of bismuth or sulfur were found included in excess of the metal that was unable to crystallize. Crace-Calvert and Johnson believed that preparing an alloy with a definite composition would allow developing a better method and quality of the product. They reasoned that the irregularities observed in commercial alloys were due not only to the presence of an excess of one of the components but also to the combination of this excess with a definite alloy, with the resulting decomposition. Thus, if a low melting metal were in excess, it would remain liquid for a long time and combine with the last portions of the true alloy, forming a new alloy of different composition. If a high melting point metal were in excess, it would be the first to solidify in the melted mass and oppose its homogeneity (Crace-Calvert & Johnson, 1855).
Crace-Calvert and Johnson wrote that the purpose of their work was not to examine the available alloys but to describe the physical and chemical properties of some new types they had discovered in which the components were present in defined proportions: (1) iron and potassium, (2) iron and aluminum, (3) aluminum and copper, (4) iron and zinc, and (5) copper alloys. For example, the iron-potassium alloy was prepared by heating in a crucible a mixture of 12 equivalents of iron filings with 8 equivalents of potassium bitartrate. The result was an alloy of composition similar to a sesquioxide, Fe2O3. After exposure to high temperature it transformed into a button containing, by weight, 74.60% iron and 25.40% potassium, corresponding to the formula 4Fe + 1K. This alloy had the same appearance as malleable iron and could be forged and welded, and was notably hard. Crace-Calvert and Johnson prepared another alloy with the same initial mixture plus some powdered wood carbon. This new alloy contained, by weight, 81.42% iron and 18.58% potassium, corresponding to the formula 6Fe + 1K. A third alloy was prepared by heating to a high temperature a mixture of iron filings with a quantity of potassium carbonate containing the same amount of potassium as in the potassium tartrate, that is, 437 part of iron + 324 of potassium carbonate. This mixture did not result in an alloy.
The potassium-aluminum alloy was prepared by heating to white red during two hours a mixture of 6 equivalents of aluminum chloride (396 parts), 40 equivalents of fine iron filings (1120 parts), and 8 equivalents of lime (224 parts) (added to eliminate the chlorine from the aluminum chloride as fusible calcium chloride). The resulting alloy contained one equivalent of aluminum (9.09%) and five equivalents of iron (90.91%). This alloy was accompanied by a button containing 4 equivalents of iron and 1 of aluminum; the same composition as one of the alloys of iron and potassium; it was also very hard and rusted in the presence of humid air. Crace-Calvert and Johnson examined the constant deposit formed at the bottom of the metallic bath of zinc and tin used to galvanize iron and found it to be composed, by weight, of 6.06% iron and 93.94% zinc, corresponding to the formula Fe + 12Zn. This alloy had a crystalline texture and was very hard and fusible. Examination of the bath itself led to the discovery of the three new alloys of tin and zinc: Sn + 11Zn (upper part), Sn + 16Zn (middle section), and Sn + 19Zn (lower part). Crace-Calvert and Johnson also prepared a series of new alloys having copper as the predominant component (Crace-Calvert & Johnson, 1855).
Crace-Calvert and Johnson published a series of papers describing some physical properties of metals, alloys, and amalgams: thermal expansion (Crace-Calvert & Johnson, 1858c; Crace-Calvert & Lowe, 1859-1860; Crace-Calvert, Johnson, & Lowe, 1861), thermal conductivity (Crace-Calvert & Johnson, 1858b, 1859a, 1860-1862); hardness (Crace-Calvert & Johnson, 1859b); specific gravity (Crace-Calvert & Johnson, 1859c), etc.
Thermal conductivity
The first publication described the measurement of the thermal conductivity of a series of metals and alloys composed pure metals combined in atomic proportions (Crace-Calvert & Johnson, 1858b). The results for metals indicated that the thermal conductivity varied according to the molecular structure. The conductivity of laminated metals was larger than of the casted ones; for example, if the relative conductivity of silver was 1000 then that of laminated copper was 845, and casted copper 811. It also varied with the axis of crystallization: A bar of zinc casted vertically had four axis of crystallization and a conductivity of 628 (against 1000 for silver); the same bar, casted horizontally had only one axis of crystallization and conductivity 608. The property also varied when the sample contained small amounts of impurities. For example, addition of 1% of silver to 99% of gold decreased its conductivity from 981 to 840. The conductivity of copper, 811, decreased to 771, 669, and 570 upon addition of 0.25%, 0.50% and 1% of arsenic. A table was also provided of the value of the thermal conductivity of alloys of tin and lead and tin and copper in different atomic proportions (Crace-Calvert & Johnson, 1858b).
The following publication referred to the thermal conductivity of mercury and its amalgams (Crace-Calvert & Johnson, 1859a, 1859-1860). The result for mercury (54) indicated that it was the worst conductor of all the metals tested. This observation was similar to that for water, which in the liquid state had a very low conductivity. In the case of amalgams, it was in accordance with the law determined previously by Crace-Calvert and Johnson that "alloys in which there is an excess of the number of equivalents of the worse conducting metals over the number of the equivalents of the better conductor, do not sensibly differ in conducting power from the worse conductor alone (Crace-Calvert & Johnson, 1855, 1859a, 1859-1860).
An additional paper added information about the conductivity of amalgams of mercury and tin in various proportions and of mercury with 2% of silver, copper, gold, bismuth, lead, cadmium, and zinc (Crace-Calvert & Johnson, 1860-1862). The results indicated that the amalgamation of mercury with a metal having greater or less thermal conductivity had no influence on the conductivity of the amalgam itself (which remained at about 680).
Hardness
Crace-Calvert and Johnson wrote that the present method for measuring hardness consisted in rubbing one body against the other and defining as "harder" the one that scratched or indented the other (Crace-Calvert & Johnson, 1859b). Thus, diamond was harder than iron and quartz harder than tin. Crace-Calvert and Johnson believed this procedure was very primitive and did not allow quantifying the property. For this purpose, they designed and build a new instrument that satisfied these requirements, which they described in detail in their paper. They also provided two tables giving the relative hardness of metals and alloys. Their results indicated that cast iron had the highest degree of hardness compared with that of all metals and alloys. For example, the relative value of 1000 for cast iron decreased to 948 for steel, 375 for platinum, 168 for cadmium, 16 for lead, 280 for an alloy of 82.95% copper and 17.05% zinc, and 243 for the same alloy with 49.32% copper and 50.68% zinc (Crace-Calvert & Johnson, 1859b).
Specific gravity
Another paper reported the value of the specific gravity of a large number of alloys of copper and tin, copper and zinc, tin and zinc, tin and lead, antimony and bismuth, and lead and antimony. The results indicated that in alloys there was always a metal that contracted while all the amalgams expanded or had a lower specific gravity, i.e. mercury and tin, mercury and bismuth, and mercury and zinc (Crace-Calvert & Johnson, 1859c).
Action of acids on metals
According to Crace-Calvert and Johnson it would of interest to complement their work on metals and alloys by studying the action of some acids upon them. Their plan of work included the action of acids (HCl, sulfuric and nitric) upon copper, zinc, and tin, and the two alloys obtained from them, i.e. brasses and bronzes (Crace-Calvert & Johnson, 1866). Crave-Calvert and Johnson wrote that the first problems they had to resolve were the preparation of perfectly pure sulfuric acid and a few grams of zinc, and in particular, the unevenness of the action of sulfuric acid, which depended on the odd state of the surface of the metal. This led them to discover that a perfectly clean surface of zinc would oxidize after some days and change its interaction with the acid. After taking into account these phenomena the results indicated something contrary to what commonly assumed to be true: concentrated sulfuric acid did not react with zinc at ordinary temperature, it only begun to do it temperatures above 130 oC; at 150 oC the reaction was fully in action. Not only this, the strength of the reaction depended on that of the acid: upon a surface of 1 m2, SO3,HO, SO3,2HO, and SO3,3HO dissolved 125, 236.6, and 9860 g of metal, respectively. The difference was more outstanding when using acid more diluted. Within 2 hours, SO3,6HO and SO3,7HO dissolved 561.6 and 5260.8 g/m2, respectively. Another interesting result was related to the course of the reaction: with SO3,HO the metal was oxidized at the expense of the acid, with release of SO2, while with SO3,2HO and SO3,3HO the gas released was a mixture of SO2 and H2S (Crace-Calvert & Johnson, 1866). The results with copper were somewhat similar. Copper started to be attacked by SO3,HO also at 130 oC; at 150 oC the amount of copper dissolved by SO3,2HO and SO3,3HO was one-half that dissolved by SO3,HO. SO3,4HO hardly attacked the metal.
The action of nitric acid on brass and bronze depended on the concentration of the acid; acid of specific density 1.14 dissolved both metals in the ratio they were present in the alloy while acid of concentration1.08 dissolved all the zinc contained in the alloy and a small amount of copper. HCl acted on somewhat similar form on an alloy composed of 49% copper and 51% of zinc: acid of specific gravity 1.20 dissolved 97% of the zinc (Crace-Calvert & Johnson, 1866).
Picric acid
In 1856 Crace-Calvert published a paper about the therapeutic properties of picric acid, in particular, the tinting the skin (Crace-Calvert, 1856). This acid had been discovered by Jean Joseph Welter (1763-1852) while studying the action of nitric acid upon silk (Welter, 1798) and rediscovered by Chevreul during the study of the action of nitric acid upon indigo (Chevreul, 1809). The extreme bitterness of picric acid suggested its possible use in medicine, for this reason Crace-Calvert prepared a mixture of picric acid and the picrates of ammonia, iron, nickel, and zinc, and asked three physicians to try them (Crace-Calvert, 1856). These physicians promptly reported that these salts had therapeutic value due to their analogy with the corresponding salts of quinine, in particular, the picrates of iron and ammonia. Pure picric acid was not useful because it produced stomach cramps but iron picrate was found to be very useful for treating headaches; ammonia picrate was useful for treating intermittent fevers (i.e. malaria), and mixed with gallic acid and opium was very effective for treating chronic diarrhea, etc. An interesting side effect was that the skin of all patients assumed a yellow color, including their eyes, as if they suffered from jaundice. This phenomenon took 3 to 6 days to appear and disappeared once they ceased to ingest the picric medicine. One gram of medicine was enough for inducing the coloration. Crace-Calvert developed an analytical procedure for detecting the presence of picric acid in urine. This method was able to detect as little as 0.01 g of picric acid in 100 g of urine (Crace-Calvert, 1856).
Analysis of chrome ores
According to Crace-Calvert, chemists were quite aware of difficulties involved in the chemical analysis of chrome ores. His own experience had shown him that the standard methods were unable to yield the same result, even when analyzing samples of the same ore (Crace-Calvert, 1853). The present methods exhibited several difficulties hard to overcome. First, they required heating at high temperatures and for several hours, a mixture of the ore with potassium nitrate and potassium carbonate. This step required crucibles, hard to obtain, which were able to support these extreme conditions: silver crucibles did not stand the high temperatures required, and porcelain or platinum ones were damaged or dissolved. The second disadvantage was due to the high density of the ore, which caused it to drop to the bottom of the melted mixture, and, consequently, be partially reduced by the potassium salts Crace-Calvert tried and succeeded in finding a more reliable analytical procedure (Crace-Calvert, 1853).
The procedure was based in mixing a given amount of chrome ore with 3 to 4 times its weigh of a mixture of slaking quicklime with NaOH and then drying and calcining the mass. The product was mixed with one-quarter part of sodium nitrate and calcined again for two hours with agitation, to favor the further oxidation of the chrome oxide formed. Further treatment with sulfuric acid, alcohol, washing, etc. ended in a solution containing the chrome in the form of a precipitate of sodium dichromate. Boiling the salt with alcohol converted it into chromic oxide, which was analyzed by Penny's method (Crace-Calvert, 1853).