INTRODUCTION
Capsaicinoids (Figure 1), the pungent compounds of the Capsicum fruits (Chili pepper), are pseudoalkaloid amides biosynthesized in the placental tissue of Capsicum by condensation of vainillilamina and nine to eleven carbons ω-1-methyl-fatty acids by the enzyme capsaicinoid synthetase (Suzuki et al., 1981a). They have been classified by (Suzuki et al., 1981b), on the bases that the chain length of their branched chain fatty acyl moiety in two groups: CAP and DC. with even number (C10) branched· chain fatty acyl moieties (group 1); NDC, HDC and HC with odd-number (C9-C11) branched chain fatty acyl moieties (group, 2). Isotopic studies revealed that vanillylamine come from phenylalanine via the phenylpropanoid pathway, while the medium-length branched-chain fatty acids come from valine or leucine via isobutyrate or isovalerate (Suzuki et al., 1981a, b, Fujiwake et al., 1980, 1982). It has been speculated that the difference in the profile and content of capsaicinoids in fruits seems to be regulated by the specificity of the capsaicinoid synthetase enzyme (Suzuki et al., 1981b, Fujiwake et al., 1980, 1982), and by the state of maturation and the metabolic pull of their ω-1-methyl-fatty acid precursors (Suszuki et al., 1981b, Vazquez et al., 2007., Aza et al., 2011), but the biochemical steps of biosynthesis pathway of the ω-1-methyl-fatty remains obscure (Calva y Rios, 1999, Aza 2011). There are evidences that biosynthesis of these fatty acids occurs by conversion of isobutyrate or isovalerate through a still uncharacterized dehydrogenase enzyme (Suzuki and Iwai, 1984), and elongation of the carbon chain by successive addition of two carbons to the acyl intermediates bonded to an acyl carrier protein (ACP), and condensation catalyzed by a 3-ketoacyl-ACP synthase II (KAS II), process analogous to the general biosynthesis of fatty acids (Markai et al., 2002), which also involves enzymes such as keto-acyl-synthase (KAS), and a thioesterase (Curry et al., 1999; Aluru et al., 2003). Even more, there is not clear evidence for the presence of the ω-1-methyl-fatty acids, precursors of capsaicinoids, in fruits or plants of Capsicum. With the aim of stressing the knowledge on this topic, the objective of this study was to investigate by HPLC and GC/FI or GC/MS, the presence and distribution of these ω-1-methyl- fatty acids regarding to capsaicinoids content in fruits, plants, and biomass from cell cultures of several Capsicum species, regarding their capsaicinoids content.
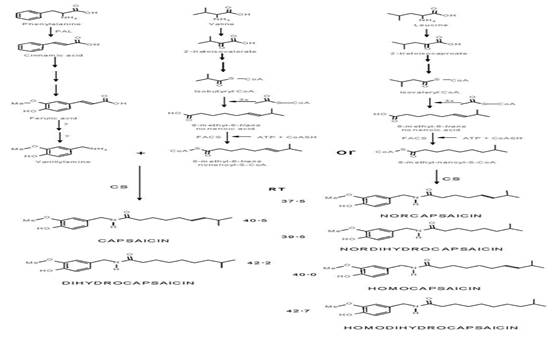
Fig. 1 Proposed biochemical pathway leading to the formation of capsaicinoids (Fujiwake et al., 1982; Yeoman et al.,1989). PAL, phenylalanine ammonia lyase; CA4H, cinnamic acid 4-hydroxylase; CA3H, coumaric acid 3-hydroxylase; CAOMT, caffeic acid O-methyltransferase; FACS, fatty acyl-CoA synthetase; CS, capsaicinoid synthase.
MATERIALS AND METHODS
Plant material in vitro and in vivo. Fruits from several Capsicum spp plants grown in greenhouse as reported in (Calva and Rios, 1999), Calva-Calva (1995), were used. The in vitro cultures of Capsicum chinense, were stablished from hypocotyl explants cultivated on MS medium supplemented with 30 g/L sucrose, 1.8 g/L phytagel, 12.5 µM 2,4- (2,4-D). and 0.5 µM kinetin (K). Cell suspension cultures were stablished from 3g of callus tissue transferred into 50 mL MS medium culture supplemented with 30g/L sucrose, 12.5 µM 2,4-D, and 0.5 µM K.
Capsaicinoids. Samples of biomass (500 mg) were ground in the presence of liquid N, extracted 3x with absolute ethanol and HPLC analysed as previously reported (Calva and Rios, 1999, Calva-Calva 1995).
Fatty acid analysis. The fatty acid fraction of the total lipids extracts from 2 g of fresh tissue ground in liquid N was trans esterified with methanol (FAME), and GC or GC-MS analyzed (Perkin Elmer Gas Chromatograph, INNOWAX column; 30m*0.32mm; the gradient used is 100°C→5°C/min→220°C-3°C min- 2°C/min →250°C-10min-end; Td 280°C, Ti. 250°C, MS or FI detector and 3 μL injection volume), as reported previously by Rivera Casado et al., (22015).
RESULTS
Growth pattern and capsaicinoids accumulation in cell suspension cultures and fruits of Capsicum spp. The growth pattern of cell suspension cultures of Capsicum spp., as that showed by the cell suspension cultures of Capsicum chinense (Figure 2A), and that reported for other Capsicum varieties, such as Capsicum frutescens (Sudha and Ravishankar, 2003a, 2003b), Capsicum annuum (Martínez Juarez et al., 2004), and Capsicum chinense (Arroyo et al., 2017), usually yield the typical sigmoid curve for the kinetic of growth of suspended plant cells (Mohamad, 2018). In contrast, the profile pattern for accumulation of capsaicinoids was usually erratic, showing presence occasionally throughout the end of the lag phase and/or the stationary phase, but most of the time these compounds are not detected along the cell suspensions growth cycle. These observations agree with previous reports for cell suspension cultures of other Capsicum varieties (Calva et al., 1995, Sudha and Ravishankar, 2003b, Martínez Juarez et al., 2004). However, in fruits, the profile pattern for capsaicinoids accumulation showed a continuous raise (Figure 2B), with a clear direct relationship with the fruit elongation and the placental development, entering to the stationary phase of accumulation when both the increment of the fruit’s length and the placental tissue growth stop, once the transition of the green color to the red, orange, or yellow mature color, characteristic of each cultivar starts (Figure 3). So, it was expected to observe the presence of the ω-1-methyl-fatty acids along with the accumulation pattern of capsaicinoids.
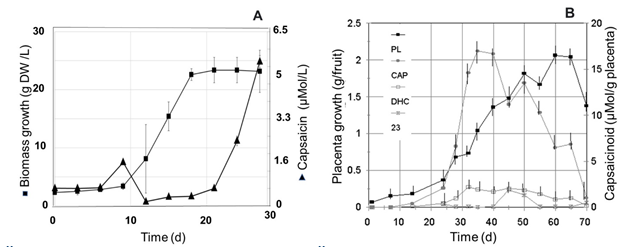
Fig. 2 A, Kinetic of growth (■) of cell suspension cultures of C. chinense (Habanero) cultivated in 125 mL Erlenmeyer flasks with 30 mL of MS medium added with 2.4D (12.5 μM ), and K (0.5 μM), on an orbital shaker at 90-100 rpm and 25°C, and accumulation pattern of capsaicin (▲), in comparison with B, the profile of capsaicin (CAP), dihydrocapsaicin (DHC), and an unidentified capsaicinoid (23), accumulation in the placental tissue (PL) of Capsicum annuum Var annuum (Chigol) fruits.
Presence of ω-1-methyl-fatty acids regarding the capsaicinoids accumulation pattern. The GC/MS chromatograms of the fatty acid fraction from the lipidic extracts of placental tissue of several Capsicum species, such as Capsicum frutescens (Piquín), and Capsicum Chinense (Habanero Kahui), and Capsicum annum (Jalapeño), were analyzed along the corresponding capsaicinoids accumulation pattern, however the presence of the ω-1-methyl-fatty acids, were not detected. But, interestingly, in the chromatograms for the extracts from immature fruits of Capsicum pubescens (Manzano), tiny amounts but showing clear signals at the retention time and molecular weight for some of the ω- 1-methyl-fatty acids precursors of capsaicinoids (7-7.5 min) were detected (inset in Figure 4A); and, the fragmentation pattern of the signal at the retention time of 7.4 minutes, corresponding to 8-methyl-6-transnonenoic acid (Figure 4A), precursor of capsaicin, produced a correlation with the fragmentation pattern of the standard fatty acid (Figure 4B), in more than 99%. Similar signals were putatively detected in extracts from leaves adjacent to the peduncle of fruits of Capsicum pubescens plants, however these results deserve further confirmation. Nevertheless, these results clearly prove the presence of the fatty acids precursors of capsaicinoids in the placental tissue of Capsicum pubescens fruits. Unfortunately, no similar results were observed for the extracts from other Capsicum species explored (Capsicum annuum, Capsicum chinense, and Capsicum frutescens), and neither for lipidic extracts from their respective cell suspension cultures.
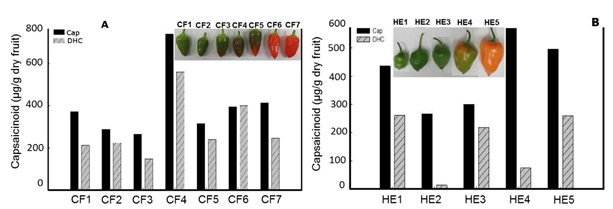
Fig. 3 Capsaicin (Cap) and dihydrocapsaicin (DHC) content in dry fruits of Capsicum frutescens (A, Piquín), and Capsicum Chinense (B, Habanero Kahui), at different stages of maturation and color.
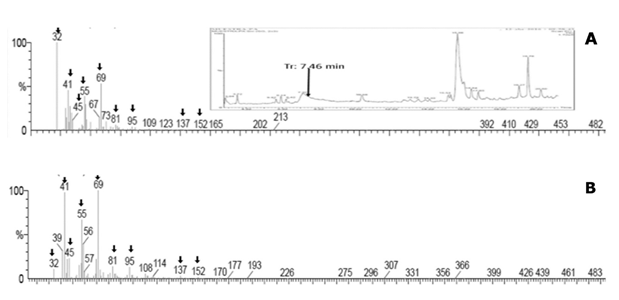
Fig. 4 A, fragmentation pattern of the signal observed at 7.46 minutes in the GC/MS chromatograms of the fatty acid fraction from the lipidic extracts of placental tissue of Capsicum pubescens fruits (inset in a), in comparison with B, the fragmentation pattern of the true 8-methyl-6-trans-nonenoic acid with m/z 170 Uma, eluting at the same retention time as the selected peak.
CONCLUSIONS
The highest accumulation of capsaicinoids occurred along the color transition of fruits: from green to the final color of the fruit, according to each specific Capsicum species. The clear presence of 8-methyl-6-trans-fatty acid detected in the placental tissue of Capsicum pubescens fruits provide evidence for these fatty acids to be the capsaicinoids precursors. However, the biochemical reaction for the biotransformation of valine and leucine into the respective fatty acids is still pending of further research.














