Introduccion
Life and career (Anonymous, 1873; Anonymous, 1873-1874, Anonymous, 1875)
Frederick Crace-Calvert (1819-1873) (Figure 1), a British chemist, was born in London on November 14, 1819, the son of Sarah Ann Trery and Alfred Crace (1782-1847), a Colonel in the British Army who added his wife's name on marriage.
No information seems to be available about Frederick's early education. In 1835, at the age of sixteen, he left London and went to France where he commenced the study of chemistry first in a private laboratory and then under Jean Pierre Girardin (1803-1884) professor of industrial chemistry at Université de Rouen. After two years he left for Paris and continued his studies at the Jardin des Plantes, the Sorbonne, Collège de France, and École de Médicine. He paid the cost of his education with the many honors and awards he received. In 1840, at the age of 21, he was appointed manager of the chemical works of the famous chemists Pierre Jean Robiquet (1780-1840) and Pierre-Joseph Pelletier (1788-1842), and lecturer of chemistry at the Gobelins. In 1846 he left this position to become préparateur of chemistry applied to the arts and manufactures under Michel Eugène Chevreul (1786-1889). While there, he published his first paper where he presented a new procedure for separating quinine and cinchonine from quinidina (Crace-Colbert, 1841). In 1843, together with Etienne Ferrand, he published a paper about the chemistry of vegetation, which was an attempt to understand the phenomenon of photosynthesis and the conversion of CO2 (Crace-Calvert & Ferrand, 1843). This publication was followed by a series of papers about the physical and chemical properties of lead (Crace-Calvert, 1843, 1845, 1846). In 1846 he returned to England to become chair of the honorary professorship of chemistry at the Royal Institution, in replacement of Lyon Playfair (1818-1898). Some time later he was also appointed lecturer at the School of Medicine in Pine Street, Manchester, a position once held by John Dalton (1766-1844). In 1847 he was elected member of the Literary and Philosophical Society of Manchester. Crace-Calvert was also a fellow of the Royal Society and the Chemical Society, a member of the Society of Arts, of the Royal Academy of Turin, and of the Imperial Academy of Saint-Petersburg, and honorary member of the Société Chimique de Paris. After several years of active research in a variety of subjects, in 1859 he established a chemical factory (first Crace-Calvert and Company and then Crace-Calvert and Thomson) where he devoted much of his activity to the manufacture and promotion of phenol as an antiseptic. It may well be said that Crace-Calvert, single-handed, was the leading person who led to the wide use of this chemical in therapeutics, sanitation, and industrial chemistry. In 1873 Crace-Calvert was appointed as one of the jury of the Vienna Exposition; unfortunately during this activity he contracted typhoid, which led to his early death in Manchester, on October 24 of the same year.
Scientific contribution
Crace-Calvert wrote about 110 papers and books (i.e. Crace-Calvert, 1876) on the subjects of inorganic, organic, and biochemistry, materials science, coal chemistry, manufacture of steel, etc. In addition to the subjects described below, he studied the chemical and physical properties of many derivatives of lead (Crace-Calvert, 1843, 1845, 1846); the production of steel (Crace-Calvert, 1852, 1861a b, 1867c, 1870; Crace-Calvert & Johnson, 1858); developed a new analytical method for analyzing chrome minerals (Crace-Calvert, 1853) and improvements in the manufacture of starches (Crace-Calvert, 1859); presented and discussed the different types of known poisons (Crace-Calvert, 1860b), the color laws of Chevreul (Crace-Calvert, 1854-1858); the physical and chemical properties of metals, alloys, and amalgams (Crace-Calvert & Johnson, 1855, 1859a b c, 1859-1860, 1860-1862, 1861,1866); etc.
Quinine and cinchonine
Crace-Calvert opened his first scientific paper with the remark that the yields of the extraction of the bases quinine and cinchonine with HCl and lime were quite capricious, even when using the same sample of bark. He now reported that he had developed a new process, exempt of the errors of the standard one, which he expected, would allow obtaining these substances at a lower price. He had found that quinine was quite soluble in a solution of calcium chloride and in a solution of limewater. Since these salts were normally used for precipitating the bases their solution in HCl, from the bark, it was almost impossible to avoid their re-dissolution, even when using an excess of lime or a lower quantity (Crace-Calvert, 1842). In his first experiments, he had studied the possibility of using aqueous ammonia and KOH as extractants and found that they suffered from the same shortcomings as the standard ones. He then precipitated a mixture of quinine sulfate and cinchonine sulfate with an excess of aqueous NaOH and found that the two alkaloids were insoluble in NaOH. Treatment of the filtrated precipitate with a solution of NaCl, followed by washing with water, resulted in the complete solution of quinine and none of the quinidine. On the basis of these results, Crace-Calvert recommended avoiding the use of lime for extracting the quinine alkaloids. Instead he suggested treating the quinquinas with an excess of HCl, followed by neutralization with sodium carbonate of the excess acid in the concentrated liquor. Once the neutralization point had been reached, the quinine and cinchonine were precipitated and separated by addition of NaOH until the liquor became slightly alkaline. Additional experiments showed that 100 parts of limewater dissolved 0.0466 part of quinine (Crace-Calvert, 1842).
Vegetable chemistry
In 1844 Crace-Calvert and Ferrand reported their results on a work intended to determine by means of chemical analysis the changes that experimented the air naturally enclosed in a vegetable, according to the organs where the change was taking place and the circumstances under which took place the principal phenomena of vegetation (Crace-Calvert & Ferrand, 1844, 1845). The pertinent memoir was divided in three sections. In the first one they discussed the possible values of the experiences carried out to prove that plants decomposed CO2 under the influence of sunlight. For example, Nicolas Théodore de Saussure (1767-1845) had conducted his experiments with vegetable enclosed in a glass bell (Saussure, 1804). According to Saussure, his experiments indicated: "green plants exposed to the successive action of day and night went through alternative inspiration and respiration of oxygen mixed with CO2. The oxygen inspired was not assimilated immediately, during inspiration it metamorphosed into CO2 that plants partially decomposed during expiration. In this manner plants were able to assimilate the oxygen that served them as atmosphere" (Saussure conducted these experiments on Cactus opuntia) (Saussure, 1804). According to Crace-Calvert and Ferrand, this setup was inappropriate for appreciating the true phenomena; the water present inside the bell saturated the air making transpiration impossible. The CO2, an indispensable food, was present in a very limited amount and was not replaced. The air expired changed its composition and led to possible pathological damage of the vegetable, manifested as changes in color and fall of leaves. No consideration was given to the lack of light and the fact that the roots were not in contact with soil, which deprived the plant from receiving indispensable substances. All these facts resulted in a disruption of the equilibrium and harmony of the vegetable functions and the inevitable appearance of experimental errors. Saussure experiments on periwinkle proved that CO2 was completely absorbed but the fact that part of the oxygen was retained and an equal amount of nitrogen was produced was no proof that the gas was decomposed into carbon and oxygen (Crace-Calvert & Ferrand, 1844, 1845).
The second of part of the memoir described the results of the experiments conducted in order to determine the chemical changes that took place in the air contained in the seedpods of the leguminous shrub Colutea arborescens (bladder senna) (Crace-Calvert & Ferrand, 1844, 1845). This was a very smart selection because these pods were known to be impermeable to atmospheric air, allowing to study the changes experimented by CO2 in the plant in its natural growing state, without the introduction of artificial factors, at different hours, under darkness, diffuse light, or sunlight, and at different stages of development of the plant. For the latter purpose, Crace-Calvert and Ferrand selected the most significant stages of the plant: young, intermediate, and old pods. The seedpods of Colutea came to maturity after one month, hence young referred to pods one week old, intermediate to 2-3 weeks, and old, pods before dehiscence when they had become transparent and almost dry, and the seeds were colored. After collection, the pods were burst under mercury, inside glass bells prepared for this purpose. The moisture of the gas was eliminated by means of sulfuric acid. The CO2 content of the dried gas was determined by bubbling it through wash bottles containing titrated KOH. The oxygen content was determined by eudiometry. The corresponding pressure and temperature were always registered, to carry out the pertinent corrections. The first table of results reported, for each stage of development, the time of sampling, the state of the sky (i.e. night, cloudy, sunny, etc.), the oxygen and CO2 content (% volume), and the total of both gases. The results indicated that the air of the seedpods was richer in CO2 than atmospheric air and that the CO2 content was higher during the night than during the day. Taking the extreme values, the amount of CO2 present at 23 hours (2.746) was almost double the one present at the time of maximum intensity of light (1.419). This table also allowed following the gradual decrease of CO2 from the night to the time it seemed to stop. It also showed that the decomposing power of the light increased with its intensity, and the duration of its action, either during the hours of a given day or by comparing the results for a cloudy day or under full sunlight. The CO2 content increased with the age of the pod in the order young < intermediate < old. The average value of the sum of oxygen + CO2, was more or less constant (23%) for the three stages of development. It was also seen that CO2 always displaced the nitrogen, sometimes some of the oxygen, but the latter did not exist as long as the amount of CO2 was large (Crace-Calvert & Ferrand, 1844, 1845).
The third part of the memoir contained the chemical analysis of the air enclosed in the lacunas of a certain number of hollow stems collected from planted bushes [Heracleum sphondylium (hog weed), Angelica archangelica (wild celery), Ricinus communis (castor bean), Dahlia variabilis (dahlia), Arundo donax (giant cane), Leycesteria formosa (Himalayans honeysuckle), and Sonchus vulgaris (milk thistle)]. This gas was immediately bubbled through the train of vessels described above. The oxygen content was determined by eudiometry. All the necessary precautions were taken to avoid mixing the gas collected with the external air (Crace-Calvert & Ferrand, 1844, 1845). The outcome was presented in in two tables giving, for each plant, the amounts of CO2 and oxygen (% volume) collected during the day and during the night, and their sum. The results indicated that the composition of the air contained in the stems was quite different from that of atmospheric air, particularly, the large content of CO2, which increased with the force of vegetation. The CO2 content during the night was larger than that during the day although in a smaller proportion than that in the seedpods. A possible explanation of this result was that the ascending and descending caudexes, as well as the roots, contributed to the absorption, while the decrease was produced by the ascending caudex where the surface was exposed to the decomposing action of the light. Another important observation was that during the night the oxygen present in the stems increased with the CO2, contrary to what occurred in the seedpods. Crace-Calvert and Ferrand also wrote that the air enclosed in the vegetables contained ammonia, a result suggesting that the ammonia extant in the external air contributed to the presence of combined ammonia in the plant (Crace-Calvert & Ferrand, 1844, 1845).
Crace-Calvert and Ferrand reached the following conclusions: (1) the seedpods of Colutea arborescens were essentially impermeable to atmospheric air; (2) vegetables decomposed CO2 under diffuse light and sunlight, (3) the decomposition rate of CO2 was proportional to the intensity of light and the duration of this factor; (4) the CO2 that disappeared in the seedpods under the influence of light rays was completely decomposed into carbon and oxygen; (5) the absorption of CO2 by plants was proportional to the force of vegetation; (6) the air in the stems did not experiment the same modifications as that in the fruits because during the night the oxygen and CO2 increased simultaneously; and (7) in vegetables, ammonia was present in the gaseous state (Crace-Calvert & Ferrand, 1844, 1845).
Putrefaction
According to Crace-Calvert, the physician J. A. Ransome had persuaded him to investigate the nature of the products released by putrid wounds in the hope of understanding the contagious effects of gangrene. This led to a series of publications on the subject (Crace-Calvert, 1860a, 1870a b, 1871a-g, 1872a b c, 1873a b). In this first exploratory work he mixed about 10 kg of meat and fish with pumice stone and disposed it in layers inside a small barrel having a lid perforated in two places, one for admitting air and the other for a tube penetrating the charge down to the bottom (Crace-Calvert, 1860a). The latter was connected to two washing bottles containing platinum chloride, in series with an aspirator. This arrangement permitted the air to sweep the putrefaction products out of the barrel and pass through the washing solution. The resulting yellow amorphous precipitate was found to contain carbon, hydrogen, and nitrogen, and, surprisingly, also sulfur and phosphorus. The analytical results indicated that a new volatile alkaloid was released and that the elements N, S, and P were not eliminated as such but as ammonia and hydrogen sulfide. Crace-Calvert also remarked that calcination of the platinum salts resulted in the release of acid and alkaline vapors having a very repugnant and nauseating odor, quite similar to the putrefaction odors, together with a white crystalline sublimate, which was found not to be ammonium chloride (Crace-Calvert, 1860a).
The next experiments were devoted to determine if the germs of fermentation and infection could be dispersed away from their source by a current of atmospheric air and infect a fluid capable of experiencing a similar change (Crace-Calvert, 1870a). In the first stage Crace-Calvert looked for the opposite result: how to deprive the infected air of this capability. For this purpose he streamed very slowly about four liters of air through a 60-cm long tube filled with cotton wool and then through another tube, 15 cm long or 45 cm long, filled with red-hot pumice stone. The two streams of air were then bubbled slowly through water deprived of animal or vegetable life. Microscopic examination of a drop sample of the water indicated that after one hour the samples of both experiments showed no signs of life; afterwards the water of the first experiment started to show an increasing quantity of microorganisms while the second sample remained inanimate until 15 days had elapsed. The second series of experiments were conducted with air thus sterilized. The purified air was first bubbled through about 500 mL of a fluid experimenting alcoholic, acetic, or butyric fermentation, or through putrid meat, and then through pure water, or a weak solution of sugar or albumen. Microscopic examination indicated that the air passing through the alcoholic or acetic fermentation bed originated a very small quantity of living microorganisms in the sugar solution and a very large one in the albumen solution. According to Crace-Calvert, these results indicated that the germs belonging to the vegetable kingdom could not be conducted any distance by the air in motion, while those of the animal kingdom were easily transported (Crace-Calvert, 1870a). Similar results were found upon exposure of the white of a new laid egg mixed with sterile water and exposed to the atmosphere for only minutes (in summer). An experiment in which white albumen was added to sterile water showed the appearance of life in five days. It was clear then that albumen facilitated the development of life (Crace-Calvert, 1871b c).
A following paper described the influence of various disinfectants in preventing the decomposition of organic substances. Crace-Calvert wrote that disinfectants were of two classes: those that destroyed by oxidation the infecting organic substance (i.e. potassium permanganate, bleaching powder, nitric acid, etc.), and those that acted by their presence, undergoing no decomposition but appearing to poison or neutralize the germs of disease (i.e. camphor, phenol, SO2, etc.) (Crave-Calvert, 1870b). In his experiments Crace-Calvert mixed solutions of albumen and starch paste with various amounts of substances commonly assumed to be antiseptic, such as phenol, chloralum (crystals or solutions of aluminum chloride hexahydrate) zinc chloride, calcium chloride, potassium permanganate, tar oil and cresylic acid, and registered the amount of time required to develop an offensive odor in the temperature range 21o to 27 oC. His results indicated that only phenol and cresylic acids were true antiseptics. These two chemicals continued acting until the albumen and starch pastes dried up. With potassium permanganate the pastes became stained after 2 days and putrid after 4 days. The corresponding figures for tar oil were 16 and 15 days, respectively (Crave-Calvert, 1870b).
Crave-Calvert also analyzed the possibility of using heat as an antiseptic tool (Crace-Calvert, 1871a d). Clearly, certain forms of life could subside when exposed temperatures up to the beginning of carbonization of organic matter. Actually, the supporters of the theory of spontaneous generation believed that protoplasmic life could not exist above 100 oC. To test this point Crave-Calvert introduced solutions of sugar, hay infusion, gelatin, and water, which had been in contact with putrid meat, inside tubes made of very thick and well-annealed glass, and then left them alone for sufficient time to assure that microorganisms had developed. The tubes were then sealed, gradually heated in an oil bath to the desired temperature level (93o, 149o, 204o, and 260 oC), and examined microscopically after four days (Crace-Calvert, 1871a d). The results indicated that in the solution that had not been heated significant signs of life were present at 100 oC and observed to decrease rapidly at the higher temperature levels. No traces of life were present at 260 oC. Similar results were found for the sugar and hay solutions. The microorganisms present in the gelatin solution were of a different kind; nevertheless, at 149 oC they had disappeared completely. Parts of the putrid meat that had been heated were mixed with albumen to test the possibility that it was still able to propagate life. This was found to be correct up to 204 oC; above this level all life had been destroyed. Similar results were obtained with putrid meat liquor that had been previously cooled down to -17 oC (Crace-Calvert, 1871a d).
An interesting series of experiments were conducted to test the effect of heat on microorganisms that that been previously dried (Crace-Calvert, 1871e). These experiments were performed on putrid fluid that had been allowed to dry in air for 24 hours, and upon putrid fluid that had been dried in the air and then completely desiccated in a stove. Four tubes from each series were not heated and used as blank tests. The remained tubes were placed in an oil bath and heated during 30 minutes to the desired temperature level (38o, 93o, 149o, 204o, 260o, and 316 oC). All the results indicated that heating to 204 oC and above, resulted in the destruction of all forms of life (this publication contains detailed tables of the results for the two series of experiments) (Crace-Calvert, 1871e).
Similar series of experiments were conducted on the action of heat of protoplasmic life dried on in cotton fabrics such as ordinary grey calico, treated chemically, washed until all free from any sizing material, and dried. Crace-Calvert wrote that the cloth was then immersed in a solution of putrid albumen, wrung out, dried at room temperature, and then sealed in tubes heated for half-an hour to the temperature levels indicated above. The fluids were then examined microscopically (Crace-Calvert, 1871f). The results showed that life was not destroyed in the fabric at 149 oC but was at 204 oC. To Crace-Calvert these results confirmed those obtained previously by Chevreul, mainly, that a much temperature was required to render albumen insoluble when dried first than when in its natural state. Examination of the calico samples, after being heated to the indicated temperatures, showed that the cloth remained stable up to 93 oC but at 149 oC it had become completely spoiled for practical purposes; at 204 oC it had mostly charred and crumbled easily into powder when rubbed between the fingers. According to Crace-Calvert, this meant that the temperature that would not destroy germ life was sufficient for damaging cotton fabric; hence, there was no benefit in employing public stoves for destroying germ life and contagion (Crace-Calvert, 1871f).
Three papers of Crace-Calvert compared the action of several substances used to prevent the decomposition of organic substances (Crace-Calvert, 1871g, 1872a b). The first paper discussed again the confusion that existed on the use of the terms antiseptic, disinfectant, and deodorant (Crace-Calvert, 1871g). Crace-Calvert explained that deodorant (i.e. manganese chloride or ferrous sulfate) was a substance that removed disagreeable or harmful odors while an antiseptic was a substance (i.e. mercuric chloride, zinc chloride, sodium chloride, arsenic acid, certain essential oils, phenol, and cresylic acid) that prevented the fermentation or putrefaction of a body. Disinfectants were to two classes: those that acted by oxidation (i.e. potassium permanganate, calcium chloride, and nitric acid) destroying the organic substance caused infection, and those that acted by their presence (i.e. camphor, SO2, and phenol), without decomposing, and apparently poisoning or turning inoffensive the germs of illness. In order to illustrate these different situations, Crace-Calvert carried a series of experiments in which he mixed, in an open flask, solutions of albumin or flour paste with various amounts of one of the substances recommended as antiseptic. He presented his results in a table listing, for each of the two solutions, the amount of antiseptic added and the time elapsed until the appearance of obnoxious odor, in the temperature range 30o to 40 oC. The results were very clear: phenol and cresylic acid were the only true antiseptics. If the only purpose was to dissipate a bad odor, it was enough to use manganese chloride, calcium chloride, ferrous sulfate, or potassium permanganate. In addition, it was known that the products of putrefaction facilitated the decomposition of substances of the same nature (the surrounding air served as germ carrier). For this reason, Crace-Calvert carried another series of experiments to determine which of the substances mentioned had the highest capability of destroying this kind of germs and avoid the putrefaction of animal substances. The results indicated that the best agent was potassium permanganate and phenol and cresylic acid, the worst ones (Crace-Calvert, 1871g).
In two following publications (Crace-Calvert, 1872a b), Crace-Calvert reported the results for similar experiments in which 26 g of a solution of albumen containing one part of egg white to four parts of distilled water, were mixed with one thousand part (0.026 g) of one of many substances (i.e. acids, alkalis, chlorine compounds, sulfur compounds, phosphates, potassium permanganate, phenol cresylic acid, phenylsulfonates, quinine sulfate, picric acid, pepper, turpentine, and charcoal). These mixtures were kept at room temperature (12.5o to 15.5 oC) and the variable observed was the number of days required to develop fungi and vibrio (Gram negative bacteria). The results indicated that the substances tested could be classified into four distinct groups: (1) those preventing the development of protoplasmic and fungus life (phenol and cresylic acid); (2) those preventing the production of vibrio life but not the appearance of fungus life (zinc chloride and mercuric chloride); (3) those allowing the formation of vibrio life but preventing fungus life (lime, quinine sulfate, pepper, turpentine, and hydrogen cyanide); and (4) those not preventing the appearance of either protoplasmic or fungus life (the remaining 25 substances). Some interesting results were that acids did not prevent the production of vibrio life but had a strong tendency to promote the growth of fungi. Alkalis did not encourage the appearance of fungi but encouraged that of vibrio (Crace-Calvert, 1872a).
Crace-Clavert conducted a series of complementary experiments in which he added to a solution of albumen, crowded with microorganisms, one-thousandth part of the substances listed above, and examined the results produced immediately after addition, and then after 1, 6, and 16 days. All the specimens were maintained at temperatures between 15o and 18 oC (Crace-Calvert, 1872a). His results indicated that cresylic acid almost destroyed the locomotive power of the vibrio immediately and prevented their regaining it during all the observation period. Phenol, quinine sulfate, zinc chloride, and sulfuric acid exhibited similar behavior but afterwards, a few individuals were seen swimming. Picric acid and phenylsulfonate initially injured the vibrio but after some time they gradually increased their number. Aluminum chloride, SO2, and hydrogen cyanide initially also injured the vibrio but after 16 days their number was the same as in the original sample. Lime, charcoal, potassium permanganate, sodium phosphate and ammonia acted in an opposite manner: they favored the production of microorganisms and promoted putrefaction (Crace-Calvert, 1872a).
A following paper added more experimental information about the influence of protoplasmic life on putrefaction (Crace-Calvert, 1872c). Crace-Calvert mentioned that he had already shown that no protoplasmic life appeared when the albumen of a freshly laid egg was added to pure distilled water, without contact with the atmosphere. Yet, exposing the solution to the atmosphere for 15 to 45 minutes resulted in the appearance of globular bodies (monads), having independent motion. The time required varied according to the season, the humidity of the atmosphere, and the temperature. The rate of impregnation seemed to be proportional to the surface exposed (Crace-Calvert, 1872c). In one particular series of experiments Crace-Calvert showed that oxygen was necessary for the existence of vibrio life. To substantiate this claim, he enclosed a solution of albumen in potable water in five glass tubes; one of them was left open to the atmosphere for 24 hours and then sealed. Crace-Calvert bubbled oxygen, hydrogen, nitrogen, and CO2 through the other four tubes, sealed them hermetically, and kept them sealed for 27 days. Inspection of the content showed that the tube containing oxygen rapidly became turbid, then the one containing air, while the other three remained clear. He also found that microorganisms released CO2 and other gases, and that these substances impaired their life when maintained in a closed environment (Crace-Calvert, 1872c). He also noted that the monads slowly developed into vibrio and then into microzyms, He described this process as follows: "a few hours after impregnation, the monads having a diameter of about 1/128000 of an inch, turned into masses, some of then turning into vibrio. The mass then broke up and each vibrio, of diameter about 1/20000 inch, attained independent movement. The vibrio grew to about 1/6400 inch and then turned into cells (which Crace-Calvert named microzyms). These cells divided into two independent parts, which repeated the process until nothing than cells were present". Crace-Calvert remarked that the cells had such swimming power that they rapidly passed over the microscope field. This stage was accompanied by the formation of a swarm of small inanimate particles. Placing the microzyms in a fresh albumen solution resulted in the generation of abundant vibrio, probably because the large amount of fresh foodstuff (Crace-Calvert, 1872c).
The last two papers discussed the influence that certain gases (oxygen, nitrogen, CO2, and illuminating gas) and other substances (diluted solutions of chlorine, calcium hypochlorite, calcium sulfide, and phenol) had on the conservation of eggs (Crace-Calvert, 1873a b). According to Crace-Calvert, the action of oxygen depended on if it was dry or wet (Crace-Calvert, 1873a). Dry oxygen had no effect but when wet it led to the egg becoming covered by a white mold presenting filaments 1-cm long, which seemed to belong to Penicillium glaucum or a Mycelium. These results were significantly modified if a small hole was made at the extreme of the egg. Now, contact with dry oxygen caused the decomposition of the egg, accompanied by the generation of a large amount of nitrogen and CO2. The putrefaction of the egg was accompanied by the formation of a large amount of microorganisms and microzyms. Humid oxygen led to a larger degree of decomposition and penetration of Penicillium to the interior of the egg. Wet or humid nitrogen had no action on intact or perforated eggs. The outside surface became covered with Penicillium but the inside remained intact. Hydrogen had no action on intact or perforated eggs, but the surface became covered with light vellum. Carbon dioxide and illuminating gas presented no action and no formation of Penicillium on the surface (Crace-Calvert, 1873a). Eggs mixed in a closed flask with a diluted solution of chlorine, of calcium hypochlorite, of limewater, of calcium sulfide, or of phenol, remained unchanged; in contact with the atmosphere they became covered with a layer of Penicillium (Crace-Calvert, 1873b).
In 1870 Crace-Calvert published a short note reporting his finding that nitrogen-containing substances of the animal kingdom released nitrogen when treated with hypochlorites (Crace-Calvert, 1870d). In his experiments he put in a glass balloon of known volume, a mixture of 200 cm3 of an aqueous solution of pure calcium hypochlorite, of known composition, and 100 cm3 of a 1.5% aqueous solution of gelatin, and observed the release of a gas containing nitrogen mixed with chlorine compounds. This process took 5 hours to be completed. Similar results were obtained when replacing the gelatin by albumin, wool, and silk:
Phenol
Crace-Calvert was a strong promoter of the use of phenol in medicine and carried extensive research about its physical, chemical, and therapeutic properties (Crace-Calvert, 1863b, 1865, 1867a b, 1870c). In his first paper (Crace-Calvert, 1863b) he wrote that phenol (carbolic acid, hydrated phenyl oxide, or phenic acid) was a white substance, crystallizing as long prisms, melting at 33.9 oC and boiling at 187.8 oC, having a slight tarry and aromatic smell resembling that of wood creosote. It was totally soluble in alcohol, ether, and glycerin, partly soluble in glacial acetic acid, and slightly soluble in water. Phenol was easily prepared by treating with NaOH the fraction of tar oil distilling between 176.7o and 204.4 oC, followed by neutralization, liberation of the phenol with HCl, and distillation of the resulting upper liquid phase (Crace-Calvert, 1863b). Crace-Calvert then quoted from the paper "On the uses of carbolic acid as a remedial agent" read by Thomas Turner, senior surgeon at the Manchester Royal Infirmary, to the Lancashire section of the British Medical Association, on the uses of phenol for the treatment of a variety of illnesses, among them, relaxation of the mucous surfaces, diphtheria, ulcers, fetid ulcers, fistulas, hemorrhoids, lupus, skin diseases, dyspepsia, intestinal worms, and the arrest of putrefaction (Crace-Calvert, 1863b).
The following publication described the discovery of a new crystalline hydrate of phenol (Crace-Calvert, 1865). Crace-Calvert observed that when a solution of four parts of phenol and one part of water was cooled to 4 oC, the walls of flask became covered with fine large rhombic crystals that proved to have a composition corresponding to the ordinary phenyl alcohol combined with one equivalent of water of formula C12H5O.2HO (Crace-Calvert is using the old chemical nomenclature, with C = 6 and water = HO). This hydrate, heated to above 16 oC, gradually lost water and left a liquid boiling at 187 oC. Crace-Calvert found that both phenyl dihydrate and ordinary hydrate were neutral to litmus paper, an observation that led to the question if the ordinary hydrate should be considered an acid (as stated in most textbooks), as an alcohol, or as substance having some similar properties to glycerin. To answer this question Crace-Calvert took 100 cm3 several solutions of KOH (having densities from 1.02 to 1.06) and mixed them with increasing amounts of a 25 cm3 solution of pure ordinary phenyl hydrate (melting at 34 oC). Several of these experiments led to the formation of two liquid phases. According to Crace-Calvert these results indicated that the solubility of phenol in the alkaline solution was not due to the combination of the acid with the alkali and that the alkaline solution was only acting as a solvent of the phenyl hydrate. Similar results were obtained with phenyl dihydrate, indicating that both substances were identical bodies, with the exception that the new hydrate contained one additional equivalent of water, not as part of composition but simply as crystallization water. In other words, phenol should be considered a neutral compound and the new substance a dihydrate of the same radical (Crace-Calvert, 1863b).
In 1867 Jean-Baptiste André Dumas (1800-1884), president of the French Society for the Encouragement of National Industry invited Crace-Calvert to lecture to the society on phenol and its properties (Crace-Calvert, 1867a). Crace-Calvert initiated his talk with a background about the products obtained when coals were submitted to the action of dull heat, in a retort. These products were of four classes: (1) coal gas, which was employed for heating, illumination, and motive power; (2) water, containing ammonia and its salts, which industry purified and used in agriculture, manufactures, and medicine; (3) black tar distillates of unpleasant odor; and (4) a porous solid residue known as coke. Distillation of the tar yielded water first; then water mixed with liquid hydrocarbons lighter than water (called light tar oils), heavy oils, and the residue called pitch (asphalt or bitumen). The light oils were used for producing coal naphtha and the heavier ones used for the preservation of railway sleepers. The pitch was used for foot pavement, public walks, and a kind of concrete named patent fuel. In 1841 Auguste Laurent (1807-1853) developed an easy process for the extraction of phenol from coal tar (Laurent, 1841) based on the fractional distillation of the light oils of coal, followed by treating with concentrated KOH the fraction boiling between 160o and 200 oC. The alkaline solution floating on the hydrocarbons was separated and then neutralized with acid. This step liberated the phenol. Charles Blachford Mansfield (1819-1855) and Pierre-Alexis-Francis Bobœuf (1807-1874) improved the yield of Laurent process by replacing KOH by NaOH and using the whole light oil instead of a fraction of it (Mansfield, 1849; Bobœuf, 1867). Afterwards, Crace-Calvert and his partners (F. C. Calvert and Co.) discovered that the best method of preparing phenol was to treat commercial impure benzenes or naphthas with weak alkalis solutions; this procedure led to a fraction containing about 50% phenol. Crace-Calvert wrote that he had used this product to promote its use by the medical profession, but he had not been successful enough because of the odor communicated by the tarry and sulfurized impurities it contained. Eventually he succeeded in overcoming this and other shortcomings and was able to manufacture a more pure phenol melting at 41 oC and boiling at 182 oC. This phenol was very pure and free of objectionable odors and Crace-Calvert believed that it should always be used as a therapeutic agent for all internal and dental purposes. It could also be used for all external applications, medical or surgical, where its tarry odor was not of importance. Phenol dissolved in water yielding a colorless solution that could be used advantageously for antiseptic and disinfecting purposes in private dwellings and hospital wards. Commercially it was also sold as a mixture with cresylic acid; this mixture dissolved in water, was appropriate as an antiseptic and disinfectant for all outdoor purposes, including the prevention of spread of diseases (Crace-Calvert, 1867a).
Crace-Calvert continued his exposition with a description of the uses that phenol had found in many areas (Crace-Calvert, 1867a b). He first repeated his understanding of the terms deodorizers, disinfectants, and antiseptics. Deodorizers were all substances that did not act as disinfectants or antiseptics; they were simply employed for removing the obnoxious gases released by putrefying organic substances, without been able to stop fermentation or decay. It was known already that these gases were not the source of infection or contagion. The latter originated from microscopic organisms floating in the atmosphere. Disinfectants, such as bleaching powder, lime chloride, SO2, and potassium permanganate, acted first as deodorizers and then as disinfectants; they were employed in large amounts to oxidize organic matter and prevent it from continuing to decompose. Antiseptics, such as mercuric chloride and phenol, destroyed the sources of decay or decomposition. They were used in small amounts and fulfilled the triple role of deodorizer, disinfectant, and antiseptic. The use of phenol for this purpose had the fundamental advantage that it could not be used for illegal purposes, as mercuric chloride, and that it destroyed that causes of putrefaction (microorganisms), without acting on the organic substances. Crace-Calvert mentioned that the properties of phenol were so powerful that 1/1000 to 1/5000 parts were enough to cancel the decomposition and putrefaction capability of urine, blood, flour, feces, etc. As an example, Crace-Calvert quoted from a paper written by Joseph Lister (1827-1912): "The material which I have employed is carbonic or phenic acid...which appears to exercise a peculiarly destructive influence upon low forms of life, and hence is the most powerful antiseptic with which we are at present acquainted...Since the antiseptic treatment (with phenol) has been brought into full operation...wounds and abscesses no longer poison the atmosphere with putrid exhalations...my wards have completely changed their character...during the last nine months not a single instance of pyæmia, hospital gangrene, and erysipelas had occurred to them" (Lister, 1867; Crace-Calvert, 1867a b).
Crace-Calvert added that his firm had stimulated the use of phenol in the cure of certain frequent diseases in sheep, such as scab; in the preservation of wood, the conservation of skins, bones, anatomical preparations, as a preventive of the decomposition of various albumen, flour, starch thickeners, the production of dyes, picric acid, etc. Crace-Calvert described in detail the use of phenol in the production of the dyes picric acid and ammonium isopurpurate (murexide), and the employment of picramic acid (2-amino-4,6-dinitrophenol) in the manufacture of explosives (Crace-Calvert, 1867a).
A short note published in 1870 described the experiences of Dr. David Davis on the use of phenol during the last appearance of cholera in Bristol (Crace-Calvert, 1870c). Davis dispersed a powder composed of 15% of phenol and cresylic acid over the decomposing excrements of the people affected by cholera and washed their clothing with an aqueous solution of phenol. This resulted in seldom having a second person living in the same room to be infected and reduced the mortality in Bristol from 36 to 40 per thousand individuals, to 18 to 20. The same positive results were obtained in the treatment of typhus, typhoid fevers, scarlet fever, and smallpox. According to Crace-Calvert, a similar treatment was used during the typhus epidemic that affected the town of Terling in the Sussex County during January to March 1868. These results led the English government to establish the use of phenol as a disinfectant in the commercial and war navy, the army, hospitals, and state prisons (Crace-Calvert, 1870c). The editor of the journal added that the use of phenol as a disinfectant had been established in Paris in 1865 and in the funeral homes in 1866.
Adulteration of animal and vegetable oils
Crace-Clavert wrote that animal and vegetable oils were being used industrially in increasing amounts and that this fact had led to their adulteration in order to compete in prices (Crave-Calvert, 1854). After being requested to examine samples of commercial oils, Crace-Calvert found that the available methods for detecting adulteration of oils were too general to provide a satisfactory answer. He went on to describe some of the available procedures, for example, that of Félix Henri Boudet (1806-1878) for recognizing the presence of drying oils in olive oil (Boudet, 1832), the diagometric method of Rousseau, based on the electrical conductivity of olive oil (Rousseau, 1823), the method of Friedrich Wilhelm Heidenreich (1798-1857), based on the color change produced when adding concentrated sulfuric acid (Heidenreich, 1842), etc. The available information led Crace-Calvert to study the possibility of using diluted acids as a tool for identifying the foreign material through the coloration produced. He believed that this phenomenon could originate from two different effects: (1) foreign matters dissolved in the oils that existed in the substance from which they were extracted, and (2) the probable action of the diluted acid on the components parts of the oils themselves. For example, addition of NaOH to acidified oil provided a different result that when no acid had been previously applied. Thus, French nut oil gave a semi-saponified fluid with NaOH and a fibrous mass when treated previously with nitric acid (Crave-Calvert, 1854). Crace-Calvert explained that he had had to employ many reagents because the adulterations used were numerous and the reactions presented by organic substances were exceedingly delicate. He then went on to describe the results when applying different reagents. Thus, a NaOH solution of specific gravity 1.340 was particularly useful to distinguish fish from other animal and vegetable oils because it gave a distinct red color. This method allowed the detection of 1% of fish oil present in any of the other oils. He gave a table of the colors produced by the action of NaOH alone on several oils (linseed, hempseed, lard, rapeseed, poppy, French nut, sesame, castor, olive etc.). Use of this table allowed distinguishing a particular oil, for example, hempseed produced a brown yellow color, linseed a bright yellow color, etc. Similar results and tables were given for sulfuric acid of specific gravity 1.475, 1.530, and 1.635; nitric acid of specific gravity 1.180, 1.220, 1.330; phosphoric acid, mixtures of sulfuric and nitric acids, and aqua regia. All these essays were conducted at room temperature, mixing one volume of reagent with 5 of oil, and noting the coloration (Crave-Calvert, 1854).
Tobacco adulterations
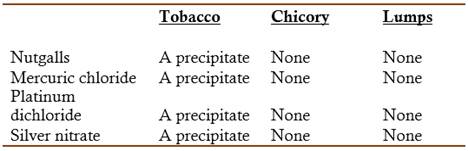
Crace-Calvert wrote that at some time he had been requested to examine four samples of tobacco, two of which seemed to be genuine and two of inferior quality and probably adulterated (Crace-Calvert, 1856). His first inspection showed that the samples did not contain molasses or sodium chloride. A carefully examination showed the presence of a small piece of iron and this suggested him the possibility that the tobacco had been adulterated with powdered iron fillings (a heavy material). A quick sweep with a magnet proved this assumption to be true: it came out loaded, to the amount of about 2 to 3% of the weight of the sample. Crace-Calvert also separated from two of the samples lumps of rare appearance and treated them with diluted HCl. The resulting solution was neutralized, concentrated by heating to a syrupy consistency, mixed with an excess of KOH, and distilled in a retort. The results indicated that the lumps were composed of a material different from tobacco and contained only a very small amount of nicotine. Furthermore, calcination of some of the lumps released an odor not characteristic of tobacco but more similar to coffee or chicory. Additional tests comparative with the alternative materials gave the following results:
Indicating that the lumps were not tobacco.
Crace-Calvert noticed that the calcination process of the suspected samples left a large quantity of ashes, insoluble in water and acid, which proved to contain chiefly sand mixed with a little of gypsum. Crace-Calvert compared these results with the ones obtained from tobacco bought for a respectable shop and concluded that the adulterated material contained almost 50% of foreign material (Crace-Calvert, 1856).
Carbon monoxide
Crace-Calvert wrote that in 1825 Chevreul published a paper where he mentioned that coloring matters were able to absorb oxygen under the influence of alkalis, and that gallic and pyrogallic were powerful agents for analyzing the atmosphere due to the rapidity with which they absorbed oxygen in the presence of alkalis (Chevreul, 1825; Crace-Calvert, 1862-1863, 1863a). This fact remained latent for many years until Justus von Liebig proved that air could be easily analyzed with these chemicals (Liebig, 1843). Crace-Clavert remarked that although many chemists considered this procedure for absorbing oxygen from a mixture to be as accurate as that of Gay-Lussac based on the employment of copper, this was misleading because a certain amount of CO was always produced during the absorption of oxygen by gallic or pyrogallic acids under the influence of alkalis. Crace-Calvert discovered this fact by accident while verifying Jean-Baptiste Boussingault (1802-1887) claim that CO was produced during vegetation under water or by the decomposition of CO2 under the influence of solar rays (Boussingault, 1863). Crace-Calvert experiments proved that this CO was not the result of the reduction of CO2 but of the action of oxygen on the pyrogallic acid that Boussingault had employed in the analysis of his gas mixtures. Crace-Calvert results indicated that 100 volumes of oxygen generated at least two volumes of CO. In addition, the production of CO also took place when atmospheric air replaced oxygen; in this situation the CO was highly diluted by nitrogen (Crace-Calvert, 1862-1863, 1863a). In a following publication Crace-Calvert added that the quantity of CO produced when one volume of oxygen was contacted with a solution containing two equivalents of sodium or potassium pyrogallate, increased significantly as the operating temperature was augmented from 15o to 75 oC (Crace-Calvert, 1864).