Introduction
The management of cardiovascular risk factors, like elevated LDL-C, is called primary prevention when patients had not previously experienced a cardiovascular event. The focalization on primary prevention on LDL reduction is based on epidemiologic data documenting a continuous, positive and graded relationship between LDL-C concentration and cardiovascular disease events and mortality, evidencing that lowering LDL-C in patients, reduces the risk in patients with or without cardiovascular disease across a broad range of concentration (Virani et al, 2020; Ference et al, 2017; Reiter-Brennan et al, 2020).
Patients without known cardiovascular disease are generally at much lower baseline of cardiovascular events risk than patients with known cardiovascular disease. The decision to recommend LDL treatment depend on the global cardiovascular disease risk because the potential absolute risk reduction by treating hypercholesterolemia will usually be smaller for patients with established cardiovascular disease (Ference et al, 2017; Reiter-Brennan et al, 2020).
End-point based studies have demonstrated a direct relationship between coronary disease and elevated serum levels of LDL-C and total cholesterol (Ference et al, 2017), as well as the benefits of lowering LDL-C on clinical end-points (Packard, 2015; Kazi et al, 2017; Kim & Le, 2020; Ford et al, 2016; Heart, 2002; Lloyd et al, 2013; Arnett et al, 2019).
Policosanol is a mixture of high molecular weight alcohols purified from sugar cane (Saccharum officinarum, L) wax (Mas, 2000) with cholesterol-lowering effects due to the inhibition of cholesterol synthesis by regulating the activity of hydroxymethyl glutaryl Coenzyme (HMG CoA) through the increase of AMP kinase activity (Singh et al, 2006; Oliaro et al, 2009; Banerjee & Porter, 2010).
The cholesterol-lowering effects of policosanol have been demonstrated in patients with type II hypercholesterolemia (Mas, 2000; Aneiros et al, 1995; Mas et al, 1999a; Canetti et al, 1997). Policosanol shows also relevant pleiotropic effects, such as the inhibition of platelet aggregation (Arruzazabala et al, 1998; Castaño et al, 1999) and the susceptibility of LDL to be oxidised (Menéndez et al, 2000a, Menéndez et al, 2000b; Castaño et al, 2002). Clinical studies and long-term post marketing surveillance studies have proven that policosanol is safe and well tolerated. (Mas, 2000; Aneiros et al, 1995; Mas et al, 1999a; Canetti et al, 1997; Arruzazabala et al, 1998; Menéndez et al, 2000a, Menéndez et al, 2000b; Castaño et al, 2002; Castaño et al, 1999; Fernández et al, 1998; Mas et al, 1999b; Fernández et al, 2004).
Talking into account the exposed background, it is logical to expect that policosanol (25 mg/day) produce benefits on lipid profile in patients with type II hyperlipoproteinemia.
Objectives: This study was undertaken to investigate the effects of policosanol (25 mg/d) administered for 12 weeks on lipid profile in patients with type II hyperlipoproteinemia, as well as safety and tolerability in this type of patients.
Materials and Methods
The study was conducted according to the principles reflected in the Helsinki statements (World Medical Association, 2013), as well as the recommendations of the World Health Organization and the Cuban regulations on Good Clinical Practices. The study protocol was approved by the Ministry of Public Health and by the Ethics Committee in Clinical Research of the Manuel Fajardo Hospital.
Study Design: This was a prospective, randomized, double-blinded, placebo-controlled study including patients after randomization treated with placebo or policosanol for 12 weeks. The study was carried out in outpatient conditions and the sample was made up of patients with type II hyperlipoproteinemia who attended a consultation at the Manuel Fajardo Hospital, Havana, Cuba.
Enrolment criteria: Patients of both sexes, aged between 25 and 70 years, with evidence of type II hyperliporototeinemia who agreed to participate in the study after signing their informed consent were recruited.
Inclusion criteria: Patients were included for randomization, if after the diet-only period, they showed total cholesterol ( 5.7 mmol/L, LDL-C ( 3.6 mmol/L and triglycerides < 4.52 mmol/L.
Exclusion criteria: Patients were excluded if active renal disease, history of liver failure, diagnosed neoplasms, alcoholism, chronic decompensated diseases. Patients who had episodes of unstable angina, myocardial infarction, stroke or any serious adverse events within the three months previous to being enrolled in the study were also excluded. In addition, also were excluded patients consuming policosanol or other lipid-lowering medications in a period of three months prior to the study, medical history of allergy to medications, or with any other special condition that at the doctor's discretion puts your health and health at risk life during the study.
Withdrawal criteria: No desire to continue for any reason, any adverse event (AE) that required it (clinical or documented by complementary analyzes), major violations of the protocol, such as lack of adherence to ingestion of treatment > 7 consecutive days and/or consumption of medications with lipid lowering action other than the study medication.
Treatment: Study medications were identical in appearance. The only difference was in the composition between policosanol and placebo tablets since in the latter, 25 mg of active ingredient was replaced by the same amount of lactose. Treatments were administered in identical packages identified by a code number and the number of treatment assigned by progressive inclusion. Study medications were randomised through a random allocation generated in the Database centre, consisting of balanced block of size ten, with a randomization ratio 1:1. Tablets must be taken once a day with dinner for 12 weeks.
Compliance assessment: Were performed from visits 3 (week 6) to 4 (week 12), compliance being assessed by patient questioning and tablet counts and defined as ( 85 % of the scheduled tablets having been consumed since the prior visit.
Concomitant medications: During the study, simultaneous consumption of medications with lipid lowering action other than the study medication was not allowed.
Assessments: Lipid profile and safety laboratory tests were performed at baseline and after 6 and 12 weeks of randomization. At each visit dietary reinforcement and physical examination were done.
Efficacy analyses
Primary efficacy variable: Significant reduction of LDL-C at the end of treatment with respect to the initial values and the placebo group. Treatment was considered as effective if LDL-C was significantly reduced by ≥ 15% (Karalis et al, 2011).
Secondary efficacy variables: Changes on other lipid profile variables (Total cholesterol, HDL-C and triglycerides) and atherogenic index (LDL-C/HDL-C and Cholesterol/HDL-C) at the end of treatment with respect to the initial values and the placebo group.
Safety and tolerability analyses: Data from physical examination (determination of body weight, pulse and arterial blood pressure), laboratory tests (glucose, creatinine, alanine amino transferase, ɤ-glutamyl transferase, alkaline phosphatase, bilirrubin, uric acid) and requests for AE were included for safety and tolerability analysis. AE were predefined as mild when did not require the withdrawal of study medication, moderate when required therapy discontinuation according to doctors’ criteria and/or specific treatment of the AE and serious when led to prolonged hospitalization, which could be fatal and non-fatal. Each AE was classified as having a causal relationship with treatment using the categories of definitely, probably, possibly, probably not, or definitively not drug-related (CECMED, 2007).
Laboratory analysis: Blood samples were drawn after 12 hours overnight fasting in the Clinical Laboratory of the Calixto García Hospital for processing and analysis. Lipid profile and laboratory test values were determined by enzymatic methods using reagent kits (Roche). Laboratory analyses were performed in Hitachi autoanalyzer. Determinations were done on the same sampling day. A quality control was performed throughout the study, so that precision (within and between-day variations) and accuracy versus reference standards were controlled.
Statistical analysis: Statistical analysis for the whole study was planned in study protocol and amendments. All data were analysed according to Intention to-treat principle. Continuous values were compared using Wilcoxon test for paired (within group comparisons) and Mann Whitney U test for independent (between group comparisons) samples. Categorical data were compared with the Fisher´s Exact Probability test. All statistical tests were two-tailed, with significance at (=0.05. Statistical analyses were performed using Statistics for Windows 10. Data management and statistical analysis was performed in the Department of Data Management and Processing of National Centre for Scientific Research.
Results
Baseline patient characteristics: Of the 65 patients recruited, 63 were eligible and randomized to policosanol (n=31) or placebo (n=32). The causes of non-inclusion were in the complementary analyzes indicated once recruited after diet period, two patients presented LDL-C< 3.6 mmol/L.
Table 1 shows the main baseline characteristics of the study patients. Both groups were well matched at randomisation. The average age of the study population was 56 years and the distribution by sex showed a predominance of 36/63 women (57.1 %) compared to 27/63 (42.9 %) of the men.
Table 1 Main baseline characteristics of study patients.
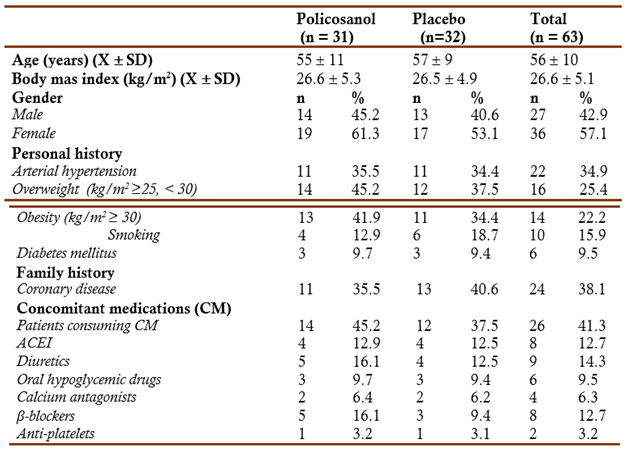
X mean, SD standard deviation, ACEI angiotensin converting enzyme inhibitors The table included CM consumed by ≥ 2 patients All comparisons were not significant
The patients included had other pathological antecedents, such as arterial hypertension (34.9 %), obesity (kg/m2 ≥ 30) (22.2 %), overweight (25.4 %), smoking (15.9 %), diabetes mellitus (9.5 %), and family history of coronary heart disease (38.1 %). The frequency of consumption of concomitant therapy (41.3 %) was similar in both groups, and in correspondence with their medical history, the consumption of antihypertensive (angiotensin-converting enzyme inhibitors -ACEI-, diuretics, calcium antagonists and β-blockers), followed by anti-diabetics and antiplatelet drugs
No patients caused withdrawal from the study. The patients included consumed all the tablets scheduled for each stage according to the count of remaining tablets and questioning the patients, which shows an excellent adherence to the treatment that was similar in both groups.
Effects on primary efficacy variable: At the end of twelve weeks of treatment, policosanol significantly reduced the LDL-C by 21.3 % with respect to initial values and by 89.2 % with respect to the placebo group (Table 2).
Table 2 Effects on lipid profile (X(SD)
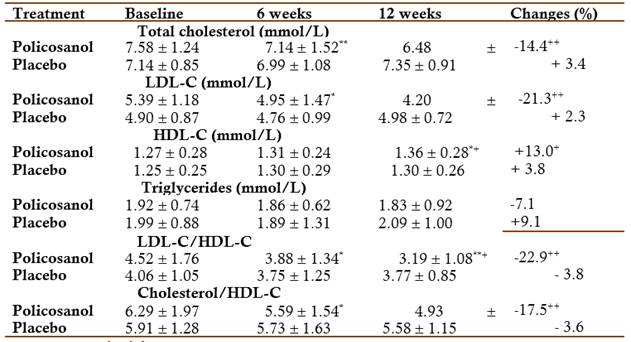
X mean, SD standard deviation
*p<0.05, **p<0.01, ***p<0.001 Comparison with baseline (Wilcoxon test)
+ p<0.05, ++ p<0.01 Comparison with placebo (Mann Whitney U test)
Effects on secondary efficacy variables: Policosanol significantly reduced the total cholesterol by 14.4 % with respect to initial values and by 76.4 % with respect to the placebo group. Treatment with policosanol increased HDL-C levels (13 %) versus baseline, differences that were also significant in comparisons made with the placebo group, while triglyceride values although reduced at the end of treatment, although this reduction was not significant (Table 2).
Policosanol significantly reduced both atherogenic index at the end of twelve weeks of treatment: LDL-C/HDL-C by 22.9 % with respect to initial values and by 83.4 % with respect to the placebo group, and significantly reduced the Cholesterol/HDL-C by 17.5 % with respect to initial values and by 79.4 % with respect to the placebo group (Table 2).
Safety and tolerability: In the analysis of the effects on the safety physical and laboratory indicators, no significant changes were obtained in any of the comparisons made, with the exception of a discrete, but significant decrease in diastolic blood pressure, which occurred in the policosanol group at six weeks of treatment (Tables 3 and 4).
Table 3 Effects on safety physical indicators (X(SD)
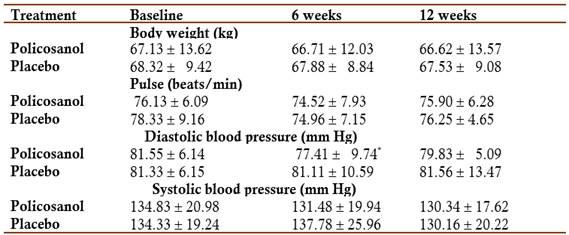
X mean, SD standard deviation
* p<0.05 Comparison with baseline (Wilcoxon test)
Table 4 Effects on safety laboratory indicators (X(SD)
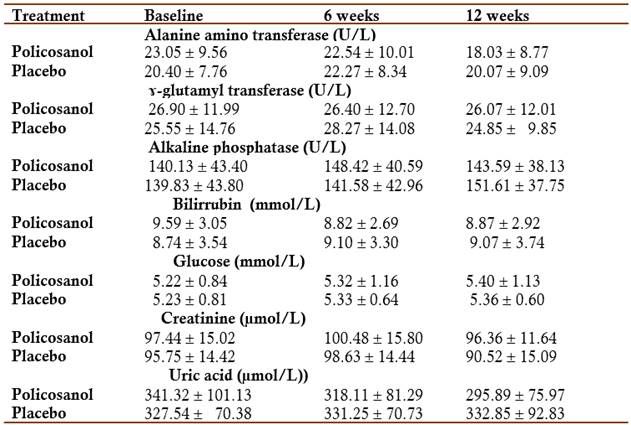
X mean, SD standard deviation
All comparisons were not significant
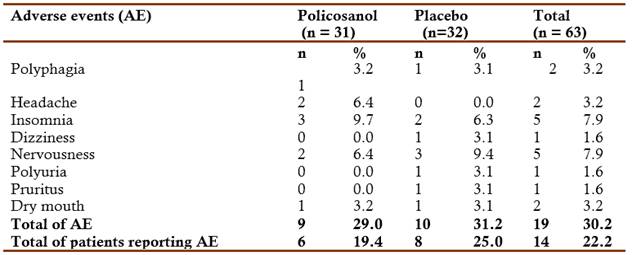
Fourteen patients reported adverse events during the study. All adverse events were mild and none of the statistical comparisons found significant differences between the groups (Table 5).
DISCUSSION
The present study demonstrates that short-term treatment (12 weeks) with policosanol (25 mg/d) in patients with type II hyperlipoproteinemia produces beneficial effects on the lipid profile and be safe and well tolerated.
Both groups were homogeneous in baseline conditions, as evidenced by the similarity of their demographic characteristics and the main response variables, which supports that the results obtained are attributable to the investigated treatment and not to disparity in the initial condition of the groups to be compared. No patients caused a withdrawal from the study.
In relation to the lipid profile, treatment with policosanol significantly reduced serum LDL-C and total cholesterol levels versus baseline and versus placebo. In addition, significantly increased levels of HDL-C versus baseline and versus placebo, while triglyceride values although decreased, were not significantly modified.
However, we must point out that although policosanol showed an efficacy profile according to its effects on lipid variables (Total cholesterol, LDL-C and HDL-C), these results were lower than those obtained in previous studies in other populations (Mas, 2000; Aneiros et al, 1995; Mas et al, 1999a; Canetti et al, 1997; Arruzazabala et al, 1998; Menéndez et al, 2000a, Menéndez et al, 2000b; Castaño et al, 2002; Castaño et al, 1999).
Treatment with daily doses of 25 mg of policosanol produced significant reductions in serum levels of LDL-C (21.3%) and total cholesterol (14.4%). These results are compatible, in a general sense, with the data obtained in a single-blind trial versus placebo in which the effects of different doses of policosanol for 8 weeks were evaluated on the lipid profile of patients with type II hyperlipoproteinemia, which included the single daily dose of 20 mg/day. However, in the present study, the decrease in LDL-C and total cholesterol values is slightly less than that obtained in the dose-effect study in which it was observed that daily doses of 20 mg administered for 8 weeks in the schedule of dinner produced a decrease in LDL-C levels of 28.9 % and total cholesterol of 19.1 % (Castaño et al, 1996).
On the other hand, in the present study there was also a significant but modest increase in HDL-C values (13%) at the end of treatment, also below that obtained with the dose of 20 mg/day, in the study dose-effect previously performed (Castaño et al, 1996).
At the end of the stage, there was a modest and non-significant average reduction in triglycerides in the treated group, while non-significant variations in these parameters were also observe in the placebo group, but their movement was practically the opposite of that of the treated group producing an increase after 12 weeks. These results are also consistent with previous studies in other populations indicating that treatment with policosanol does not produce a significant and reproducible reduction in triglyceride levels, a fact that implies that the fundamental lipid-lowering action of the product is not exerted at this level (Mas, 2000; Aneiros et al, 1995; Mas et al, 1999a; Canetti et al, 1997; Arruzazabala et al, 1998; Menéndez et al, 2000a, Menéndez et al, 2000b; Castaño et al, 2002; Castaño et al, 1999).
The reduction (in %) of the atherogenic index (LDL-C/HDL-C and Cholesterol/HDL-C) was satisfactory and behaved in a consistent way with the variations of the independent parameters (cholesterol, LDL-C and HDL-C). These data confirm that the reduction in the LDL-C/HDL-C index is greater than that of the Cholesterol/HDL-C index, similar to the movements of HDL-C and cholesterol. These data also coincide with those o f other studies, since in all cases, the results have revealed a decrease in the LD-C/HDL-C and Cholesterol/HDL-C index related to the treatment.
It was demonstrated that policosanol inhibits cholesterol synthesis in the firth step of its metabolic pathway through activation of Adenosine Monophosphate Protein Kinase (AMPK), which in turn inhibit Hydroxyl-Methyl-Glutaryl-Coenzyme A-Reductase (Singh et al, 2006; Oliaro et al, 2009; Banerjee & Porter, 2010). AMPK, once activated, also inhibit Acetyl CoA Carboxylase (ACC). The inhibition of ACC increases fatty acid oxidation and reduces lipid synthesis, protecting in this way, muscle, heart, and others tissues from lipotoxicity. In addition, AMPK activation is associated with a wide array of beneficial effects, could explain the low level of side effect and compliance in the treated group versus placebo (Cho et al, 2019).
After intestinal absorption, very long chain fatty alcohols (VLCFA) are up taken by the liver and partially converted into carboxylic acids. These results indicated that higher intake of VLCFA is significantly associated with favorable metabolic status including lower levels of circulating triglycerides. Other study confirmed that circulating serum VLCFA were independently associated with favorable profiles of blood lipids (lower triglycerides and increase HDL-C); others cardio vascular disease risk markers, and a lower cardiovascular disease risk by 52 % (Cho et al, 2019).
On the other hand, fatty alcohols are substrates for the synthesis of plasmalogens in peroxisomes, which are potent endogenous antioxidants. Plasmalogens are released from the liver as component of lipoproteins thus protecting them from oxidation, and favoring its functionability. Policosanol seems to present regeneration abilities via enhancement of HDL functionability (Cho et al, 2018; Kim et al, 2017).
Treatment with policosanol (25 mg/d) was safe and well tolerated, which coincides with previous data on the safety of policosanol in other populations and in clinical practice.15-25
Regarding the safety parameters (physical and laboratory) indicators, there were no significant changes in the variables evaluated with respect to the placebo group. Policosanol, not placebo, modestly, but significantly reduced diastolic blood pressure, consistently with some previous data (Fernández et al, 1998; Mas et al, 1999b; Fernández et al, 2004).
Fourteen patients reported adverse events during the study. All adverse events were mild and none of the statistical comparisons found significant differences between the groups.
It is concluded that although treatment with Policosanol (25 mg/d) produced beneficial effects on the lipid profile in these patients at 12 weeks, in addition to being safe and well tolerated, it is not justified to indicate this dose, due to obtaining results lower than those obtained with the 20 mg/d dose in a shorter treatment time (8 weeks).