INTRODUCTION
Algae are photosynthesizing eukaryotes that can be found in a wide range of habitats, such as in humid soils, lakes, rivers, snow and, mainly, in oceans; they always play an important role in the ecosystems in which they are inserted (Lee et al., 2017). As they are abundant and present wide diversity, algae are divided into two groups, according to their type of organization and their size, known as microalgae and macroalgae.
In addition to this division, macroalgae are also subdivided according to the main photosynthetic pigment they produce, classified as green algae (Chlorophyceae), which are those that present chlorophyll A and B; red algae (Rhodophyceae), which produce phycoerythrin; and brown algae (Phaeophyceae), which that produce the carotenoid pigment called fucoxanthin (Costa et al., 2010; El-Said & El-Sikaily, 2013; O’Sullivan et al., 2012).
Humanity has been using seaweeds for a long time to complement their diet, mainly because they possess great nutritional value. After their use for therapeutic purposes was observed, seaweeds also proved to have great relevance to medicine, making it fundamental to study their composition and potential pharmacological properties (Salehi et al., 2019).
Among their potential pharmacological actions, the antioxidant characteristic of algae has been speculated on. This is because algae present components and/or antioxidant mechanisms that guarantee their survival in their natural habitat, acting against abiotic factors that generate stress, such as salinity, exposure to sun and wind and repeated cycles of immersion and emersion (Collins et al., 2016; Irkin & Yayintas, 2018; Rocha et al., 2018; Seddek et al., 2019).
Antioxidants are compounds that prevent the formation of biological substances and reactive species, thus preventing chemical damage from oxidative stress (Melo et al., 2013). Oxidative stress acts by causing a dysregulation in cell metabolism, leading to an imbalance in homeostasis and the generation of reactive oxygen species (ROS), which react to other molecules, such as proteins and DNA (Chandrasekaran et al., 2017). Thus, antioxidant compounds have been shown to be increasingly important to medicine, as they play a significant role in several degenerative conditions and diseases common to ageing, such as in neurodegenerative diseases, inflammatory conditions and cardiovascular diseases (Chandrasekaran et al., 2017; Li et al., 2008; Powers et al., 2011).
Although an ideal antioxidant has not yet been found, researchers consider that it would have the ability to remove free radicals, chelate transition metals and be absorbable, as well as being able to interact with other antioxidants (Valko et al., 2006).
There are numerous bioactive substances found in brown seaweed that present at least one of these antioxidant properties. Some of them, such as various polysaccharides, brown algae-specific polyphenols (phlorotannins) and a peculiar type of carotenoid, fucoxanthin, will be addressed here. The present review aims to gather information regarding the composition and chemical structure, extraction and isolation protocols, as well as the in vivo and in vitro antioxidant activity of secondary metabolites found in brown algae species.
POLYSACCHARIDES
Polysaccharides are known for their various functions within organisms, such as energy reserve, maintenance of ionic balance in the cell and prevention of cell dehydration. In most brown algae, two types of polysaccharides are especially found. However, despite the wide variation in their chemical composition, both polysaccharides have sulfated L-fucose (Camara et al., 2011). The nomenclature and classification of sulfated L-fucose polysaccharides is shown in different ways in the literature. For this reason, this review will adopt the classification and nomenclature suggested by Reyes et al. (2020), in which polysaccharides composed of up to 90% of L-fucose monosaccharides are called fucoidans. Those with more than 90% fucose in their composition are called fucans (Reyes et al., 2020).
Besides fucans and fucoidans, other polysaccharides found in brown algae, such as laminarin, alginate and other secondary metabolites, such as carotenoids (fucoxanthin) and polyphenols (phlorotannins) have also been reported in the literature with antioxidant properties (O’Sullivan et al., 2010; Sellimi et al., 2018; Melo et al., 2013; Wang et al., 2019).
FUCANS AND FUCOIDANS
Chemical composition
Fucans and fucoidans are polysaccharides formed from α-L-fucopyranose, grouped in this way due to the amount of this monosaccharide presented. Fucans are composed mostly of fucose, displaying only up to 10% of other sugars in their structure, while fucoidans are heteropolysaccharides that present more than 10% of monosaccharides other than fucose (Reyes et al., 2020).
Both fucans and fucoidans are found inside cells and in the cell wall of brown algae (O’Sullivan et al., 2010) and, in addition to fucose, they also have other monosaccharides such as galactose, glucuronic acid, glucose, mannose and xylose, and sometimes even proteins (Berteau & Mulloy, 2003; Patankar et al., 1993; Rocha et al., 2001; Wang et al., 2019). In some cases, fucoidans may even contain more of other monosaccharides than fucose, e.g. galactose (Okuzumi et al., 1993).
The chemical composition of these compounds has been shown to vary according to the season of the year (Black et al., 1952), age, species (Bruhn et al., 2017), method of extraction (Mabeau et al., 1990), part of the seaweed from which it is taken (Rocha et al., 2001) and geographical location of the alga (Bruhn et al., 2017).
Chemical structure
The chemical structure and composition of these homo- and heteropolysaccharides also vary. Generally, they are branched sugars of varying molecular weight (Almeida, 2014), which do not show a regular pattern of sulphation, with possible replacement of L-fucopyranose by sulphate (SO3-) at carbons C-2, C-4 and possibly at C-3 (Ale et al., 2011). The glycosidic bonds found between residues in the main chain are α(1→3) type or alternating α(1→3) and α(1→4) bonds (Chevolot et al., 2001; Cumashi et al., 2007).
Fucans, like fucoidans, have high proportions of hydroxyl groups (-OH), which makes them hydrophilic compounds and, in most cases, soluble in water (O’Sullivan et al., 2010). This characteristic consequently makes inter- and intra-chain hydrogen bonds common in these polymers, which generates in them a certain structural rigidity (O’Sullivan et al., 2010).
Therefore, both bioactive compounds have great structural variability, and it has been observed in the literature that each extracted compound has a different structure (Ajisaka et al., 2016; Bilan et al., 2002).
LAMINARIN
Chemical composition
Laminarin is the main polysaccharide of the glucan family present in brown algae, where it is located inside the vacuoles of the cells, not belonging to the cell wall as in the case of fucans and fucoidans (Kadam et al., 2015). Despite its low molecular weight, it is composed mainly of (1,3)-β-D-glucopyranose residues (O’Sullivan et al., 2010).
Chemical Structure
Structurally, laminarin is a linear chain formed by glycopyranoses linked by β-(1,3) and β-(1,6) glycosidic bonds and a reducing end with a β-D-glucose residue (Jin et al., 2014). It does not have very branched structures (Dunstan & Goodall, 2007), although this factor, as well as the degree of polymerization and the ratio of β-(1,3) and β-(1,6) glycosidic bonds, varies according to the algae species analyzed (Kusaykin et al., 2006). Despite this variation, most laminarins, by means of intra-chain hydrogen bridges, form complex structures, which are stable and resistant (Neyrinck et al., 2007).
ALGINATES
Chemical composition
Alginate, or alginic acid, according to the Food Chemicals Codex (FCC), is a "hydrophilic colloidal carbohydrate" found both in bacteria and in the extracellular matrix of all brown algae (Davis et al., 2003). Also as described by the FCC, these are composed of residues of β-D-mannuronic and α-L-guluronic acids in the form of a pyranose ring (Nielsen et al., 2004), and, unlike fucans, do not have sulphate (Cong et al., 2014). They have been widely studied for new sources of natural bioactive compounds (Kim et al., 2010).
Chemical Structure
As described above, alginic acids are composed of blocks of β-D-mannuronic (M) and α-L-guluronic (G) acid residues, linked by bonds of the type (1=>4) (Smidsrød & Haug, 1967). Typically, residues are arranged in linear sequences of mannuronic (M-block) or guluronic (G-block) acids or even alternately (MG-block) (Smidsrød & Haug, 1967).
FUCOXANTHIN
Chemical composition
Carotenoids are organic pigments that are produced by plants, algae, bacteria, fungi and some animals (Aghajanpour et al., 2017; Fraser & Bramley, 2004). These pigments are divided into two subclasses, according to their composition, with those composed only of carbon and hydrogen classified as carotenes, and those that present substituent groups with oxygen, such as hydroxyl, keto and epoxy groups, classified as xanthophylls (Fraser & Bramley, 2004; Oliver & Palou, 2000). Fucoxanthin is an example of a xanthophyll found in brown seaweed (D’Orazio et al., 2012) and has shown great antioxidant potential in the literature (Roehrs et al., 2011; Sachindra et al., 2007).
Chemical Structure
Fucoxanthin(3'-acetoxy-5,6-epoxy-3,5'-dihydroxy-6',7'-dihydro-5,6,7,8,5',6'-hexahydro-β-carotene-8-one) is a structurally unique carotenoid that exhibits an allenic band not found in other triterpenoids (Peng et al., 2011; Takaichi, 2011). In addition, it is also composed of a 5,6-monoepoxide, two hydroxyl groups and a carbonyl group, which, as a whole, guarantee this compound various biological activities, for example, its antioxidant effect (Miyashita & Hosokawa, 2017).
PHLOROTANNINS
Chemical composition
Phlorotannins are the phenolic compounds formed from phloroglucinol (1,3,5-trihydroxybenzene) (Ragan & Jensen, 1978), found in various amounts in seaweed (Zhang et al., 2018). Many studies show that this variation in content is linked not only to the species, but also to the climate and exposure to the sun in which that species is found. Therefore, there is a difference in similar species in different countries, or that are located in different places on the coast, such as one at high tide and the other at low tide (Generalić Mekinić et al., 2019).
Chemical Structure
Phlorotannins are characterised by two or more phloroglucinol structural units, which are linked by covalent bonds between carbons or ester bonds (Generalić Mekinić et al., 2019). In addition, these compounds have structural variants that may include hydroxyl groups or linkages to other monomers (Salminen & Karonen, 2011). Some studies on the structure of phlorotannins have shown that their antioxidant activity is related to the hydroxyl groups, which change according to their quantity and location in the molecule (Agregán et al., 2017). Table 1 illustrates the secondary metabolites of brown seaweed, which have antioxidant properties already described in the literature.
Table 1. Antioxidant substances produced by brown algae species.
Antioxidant substance
Species
References
Fucan
Canistrocarpus cervicornis
Fucus vesiculosus
Laminaria japonica
Padina gymnospora
Sargassum filipendula
Fucoidan
Ascophyllum nodosum
(Albuquerque et al., 2004; Leite et al., 1998)
Cladosiphon okamuranus
Cystoseira barbata
Dictyopteris delicatula
(Albuquerque et al., 2004; Costa et al., 2010)
Dictyota cervicornis
Dictyota menstrualis
(Costa et al., 2010; Costa et al., 2011)
Dictyota mertensii
(Albuquerque et al., 2004; Costa et al., 2010)
Fucus vesiculosus
(Ajisaka et al., 2016; Corsetto et al., 2020; Wang et al., 2010)
Hizikia fusiformis
Kjellmaniella crassifolia
Laminaria japonica
(Dietrich et al., 1995; Florentin, 2015; Li et al., 2008; Prieto et al., 1999)
Nemacystus decipiens
Saccharina cichorioides
Sargassum filipendula
Sargassum fulvellum
Sargassum graminifolium
Sargassum horneri
(Ajisaka et al., 2016; Freinbichler et al., 2008)
Spatoglossum schroederi
Turbinaria conoides
(Wolfe & Rui, 2007; Yruela, 2005)
Turbinaria ornata
Laminarin
Ascophyllum nodosum
Cystoseira barbata
Laminaria digitata
(Andrikopoulos, 2010; Iwasaki et al., 2012; Tierney et al., 2013)
Laminaria hyperborea
Turbinaria conoides
Alginates
Dictyopteris delicatula
Laminaria japonica
Lobophora variegata
Turbinaria conoides
Fucoxanthin
Himanthalia elongata
Cystoseira hakodatensis
Ecklonia cava
Fucus vesiculosus
Hizikia fusiformis
Laminaria japonica
Sargassum fulvellum
Sargassum horneri
Turbinaria ornata
(Kelman et al., 2012; Kumar et al., 2008; Yruela, 2005)
Undaria pinnatifida
(Fung et al., 2013; Iwasaki et al., 2012; Kim et al., 2014)
Phlorotannins
Undaria pinnatifida
(Kim et al., 2014; Mak et al., 2013)
Cystoseira hakodatensis
Ascophyllum nodosum
Ecklonia cava
(Athukorala et al., 2007; Shibata et al., 2008; Wang et al., 2019)
Ecklonia kurome
Ecklonia stolonifera
Eisenia bicyclis
Fucus ceranoides
Fucus serratus
(Gager et al., 2020; O’Sullivan et al., 2011)
Fucus spiralis
Fucus vesiculosus
(Corsetto et al., 2020; Gager et al., 2020; Moroney et al., 2015)
Halidrys siliquosa
Himanthalia elongata
Sargassum horneri
Sargassum kjellmanianum
EXTRACTION AND ISOLATION OF SECONDARY METABOLITES
As previously discussed, there are many factors that influence the composition and yield of brown seaweed extracts, and one of them is the extraction method (Corsetto et al., 2020). It is possible to find in the literature several different protocols that are applied for the extraction and isolation of secondary metabolites with antioxidant action. Thus, the protocols with greater repercussion were selected for this review.
In the protocol described by Dietrich et al. (1995), for the extraction of alginate and fucoidan, algae of the species Padina gymnospora, Sargassum vulgare and Dictyopteris mertensis were collected on the north-east coast of Brazil and immediately taken for drying at 50ºC and subsequent trituration. The ground material was then suspended in NaCl (0.1 M) and had its pH adjusted to 8.0 by means of NaOH. To assist the proteolytic digestion, maxatase, an alkaline protease, was added to the mixture. After 24 hours of incubation, at 60ºC, with agitation and periodically adjusting the pH of the solution, filtration was performed.
At the end of filtration, an ion exchange resin was added to the filtrate and the final mixture was again stirred for 24 hours, but this time at room temperature and under a layer of toluene. The suspension was again filtered, and the resin retained by the filter was washed first with water at 50ºC and then with NaCl (0.1 M) at room temperature. In the case of the extraction of the crude polysaccharides, the washing is done directly with NaCl (3 M). The washed resin was added to a column for the elution of the acid polysaccharides with increasing concentrations of sodium chloride. Methanol was added to the fractions and then cold storage was performed for 48 hours. The material that precipitated during this period was collected, centrifuged, washed with 80% methanol and resuspended in water for analysis (Dietrich et al., 1995). It is noteworthy that this same protocol was used for other species, such as Dictyota menstrualis (Araújo, 2018) and Spatoglossum schroederi (Leite et al., 1998).
Other similar protocols are also found, such as the one reproduced by Albuquerque et al. (2004) with Dictyota menstrualis, in which instead of the crushed alga being suspended in 0.1 M NaCl, 0.25 M NaCl is used, and, after incubation for 24 hours and filtration, it is kept at 4°C and fractionated by precipitation, starting with the addition of acetone and resting for 24 hours. It is then centrifuged for 20 minutes and, after being dried under vacuum, the precipitate is resuspended in distilled water and taken for analysis. The study by Rocha et al. (2005) proceeded in an almost identical way but incubated the alga Spatoglossum schroederi for 18 hours.
Similarly to Albuquerque et al. (2004), Costa et al. (2011) performed the extraction of polysaccharides from Sargassum filipendula by changing the alkaline protease from maxatase to prozyme, while Melo et al. (2013) made a composition of the two adaptations, incubating the mixture of prozyme and NaCl (0.25 M) for 18 hours, for the extraction of fucoidan and glucan from Dictyopteris justii.
In contrast to the other methods cited so far, Wang et al. (2009) presents a procedure in which, to prepare the samples of Fucus vesiculosus, Fucus serratus, Laminaria hyperborea, Laminaria saccharina, Laminaria digitata and Alaria esculenta for the extraction of their secondary metabolites, lyophilization was performed for a period of 72 hours, after which the samples were pulverized and stored at -80ºC. To start the extraction process, in fact, distilled water or 70% (v/v) aqueous acetone was added to the powdered samples, incubating the mixture under stirring for 24 hours at room temperature. After this time, the samples were centrifuged at 4°C and then filtered. The acetone was then removed by means of rotation evaporation, and the concentrate and the supernatant of the extract were lyophilized and stored at -80ºC until the analyses, for which they were dissolved in distilled water.
Another protocol that stands out from the others consists of the preparation through freezing by direct immersion of the algae samples in liquid nitrogen. For the actual extraction, the addition of mixtures of apolar and/or semi polar organic solvents occurs. This protocol was tested by Rajauria (2019) with algae of the species Laminaria digitata, Laminaria saccharina and Himanthalia elongata, and also with four different solvent mixtures, always in equal volumes, which were hexane and diethyl ether; hexane and chloroform; diethyl ether and chloroform; and hexane, diethyl ether and chloroform. After immersion in the solvents, the samples were filtered and centrifuged and the supernatant dried and dissolved in methyl acid in the high-performance liquid chromatography (HPLC) to be analyzed. All this methodology was carried out under low light conditions to minimize the possibility of degradation of antioxidant compounds by light.
IN VITRO ANTIOXIDANT ACTIVITY
There are several methods for assessing the in vitro antioxidant activity of biologically active substances, ranging from chemical assays with lipid substrates to more complex assays using a variety of instrumental techniques and cell culture assays. This review provides a description of these different methods and in vitro assays, as well as the findings in the literature from their use.
Total Antioxidant Capacity (TAC)
This chemical assay measures the ability of the extract or molecule to neutralize free radicals, such as ROS, through electron donation. For this method, it is common to use the reduction of Molybdenum+6 to Molybdenum+5 and the subsequent formation of a phosphate/molybdenum+5 complex at acid pH (Prieto et al., 1999). Thus, usually samples of the extracts are used in diluted form and combined with a reagent solution composed of sulphuric acid, sodium phosphate and ammonium molybdate, a mixture which is kept at 90-100°C for approximately 90 minutes and then analyzed in a spectrophotometer, measuring the absorbance at 695 nm against a blank and generating a result displayed as a percentage in relation to the standard ascorbic acid (Almeida, 2014; Araújo, 2018; Florentin, 2015).
Total antioxidant capacity has already been analyzed for several species of brown algae, such as Ascophyllum nodosum (Tierney et al., 2013), Dictyota cervicornis (Costa et al., 2010), Dictyopteris delicatula (Costa et al., 2010; Florentin, 2015), Dictyopteris justii (Melo et al., 2013), Dictyota menstrualis (Araújo, 2018; Costa et al., 2010), Dictyota mertensii (Costa et al., 2010), Fucus spiralis (Tierney et al., 2013), Lobophora variegata (Almeida, 2014), Pelvetia canaliculata (Tierney et al., 2013), Sargassum filipendula ( Costa et al., 2010) and Spatoglossum schroederi (Costa et al., 2010).
Reducing Power
The reducing power, also known as Ferric reducing antioxidant power (FRAP), is the quantification of the capacity of an extract or molecule to reduce another compound from the donation of an electron. For its determination, it is necessary to incubate the solution containing different concentrations of extracts in phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide (1%) for 20 min at 50 °C. A 10% Trichloroacetic Acid (TCA) solution is then added and the absorbance is read, measured at 700 nm. As in the CAT determination, the analysis result is given as a percentage in relation to the standard ascorbic acid (Almeida, 2014; Araújo, 2018; Florentin, 2015).
This analysis has already been used for several species of brown algae, such as Alaria esculenta (Gager et al., 2020), Ascophyllum nodosum (Gager et al., 2020; Tierney et al., 2013), Bifurcaria bifurcata (Gager et al., 2020), Dictyota cervicornis (Costa et al., 2010), Dictyopteris delicatula ( Costa et al., 2010; Florentin, 2015), Dictyopteris justii (Melo et al., 2013), Dictyota menstrualis (Araújo, 2018; L.S. Costa et al., 2010), Dictyota mertensii (Costa et al., 2010), Fucus serratus (Gager et al., 2020), Fucus spiralis (Tierney et al., 2013), Halidrys siliquosa (Gager et al., 2020), Himanthalia elongata (Gager et al., 2020; Rajauria, 2019), Laminaria digitata (Rajauria, 2019), Laminaria ochroleuca (Gager et al., 2020), Laminaria saccharina (Rajauria, 2019), Lobophora variegata (Almeida, 2014), Pelvetia canaliculata (Tierney et al., 2013), Sargassum filipendula and Spatoglossum schroederi (Costa et al., 2010).
Hydroxyl radical scavenging
Besides being a good electron donor, an antioxidant can also act by sequestering reactive substances. Hydroxyl is a ROS that is directly linked to cell damage, this being the most reactive of the radicals, which makes it the most damaging. In vivo, its main production is linked to the transition reaction of metals with the superoxide ion by means of the Fenton reaction (Figure 1) (Valko et al., 2006).
Fig. 1. Fenton Reaction
For the determination of the hydroxyl radical sequestration potential, different concentrations of the alga extract are added to a solution containing phenatroline, phosphate buffer and Fe2SO4. H2O2 (15%) is added and then the mixture is incubated at 37°C for 90 minutes. At the end, centrifugation is performed, reading at 536 nm and the formula is applied: (%) = (1 - Asample /AControl) x 100, where AControl is the absorbance of the mixture in the absence of the test sample, and Asample, the absorbance of the mixture in the presence of the sample (Almeida, 2014; Araújo, 2018; Florentin, 2015).
In the literature, it is possible to identify several species of brown algae that have been analyzed for hydroxyl radical scavenging, for example, Dictyota cervicornis (Costa et al., 2010), Dictyopteris delicatula (Costa et al., 2010; Florentin, 2015), Dictyopteris justii (Melo et al., 2013), Lobophora variegata (Almeida, 2014), Dictyota menstrualis, Dictyota mertensii, Sargassum filipendula and Spatoglossum schroederi (Costa et al., 2010).
Superoxide radical scavenging
Unlike hydroxyl, the superoxide radical is considered a primary ROS, i.e., it is capable of generating other derivative reactants through direct interaction with other molecules or through processes catalyzed by metals or enzymes, and it is widely found in mitochondria (Valko et al., 2006). Like hydroxyl, superoxide is also very damaging to the body and is associated with numerous diseases such as stroke, cancer, diabetes and nerve and liver damage (Freinbichler et al., 2008).
The test performed for its determination is based on the ability of the sample to inhibit the photochemical reduction of nitroblue tetrazolium (NBT) in the riboflavin-light-NBT system (Araújo, 2018). Therefore, the reagents are added to the sample at different concentrations, and the production of formazan blue is monitored by increasing the absorbance at 560 nm after being illuminated for 15 minutes with a fluorescent lamp. This reaction is usually performed in a light dissipating chamber.
As with hydroxyl radical scavenging, it is also possible to observe the analysis of various brown algae with respect to their ability to scavenge superoxide radicals, such as Dictyota cervicornis, Dictyopteris delicatula (Costa et al., 2010), Dictyopteris justii (Melo et al., 2013), Dictyota menstrualis (Araújo, 2018; Costa et al., 2010), Dictyota mertensii, Sargassum filipendula and Spatoglossum schroederi (Costa et al., 2010).
1,1-diphenyl-2-picryl-hydroxyl (DPPH) radical scavenging
Similarly to those mentioned above, the assay highlighted here uses as principle the reduction of free radical 1,1-diphenyl-2-picryl-hydroxyl (DPPH) when the molecule analyzed is an antioxidant, thus donating an electron to the radical (Gager et al., 2020). This is measured by the formation of a purple-blue color after the sample is dissolved in 70% methanol, centrifuged and its supernatant mixed with DPPH with methanol. The absorbance reading is usually taken at 520 nm, and the DPPH sequestration effect is expressed using a mathematical formula as: [(Ablank - Acontrol) − (Asample − Acontrol)] / (Ablank − Acontrol) × 100 , in which Ablank is the absorbance of the blank, to which 70% ethanol is added instead of the supernatant of the centrifugate, and Acontrol is the absorbance of the control made with the addition of 70% ethanol instead of the DPPH solution (Araújo, 2018; Corsetto et al., 2020; Florentin, 2015).
This analysis has already been used for several species of brown algae, such as Alaria esculenta (Gager et al., 2020), Ascophyllum nodosum (Gager et al., 2020; Tierney et al., 2013), Bifurcaria bifurcata (Gager et al., 2020), Dictyopteris delicatula (Florentin, 2015), Dictyota menstrualis (Araújo, 2018), Fucus serratus (Gager et al., 2020), Fucus spiralis (Tierney et al., 2013), Fucus vesiculosus (Corsetto et al., 2020), Halidrys siliquosa (Gager et al., 2020), Himanthalia elongata (Gager et al., 2020; Rajauria, 2019), Laminaria digitata (Rajauria, 2019), Laminaria ochroleuca (Gager et al., 2020), Laminaria saccharina (Rajauria, 2019), and Pelvetia canaliculata (Tierney et al., 2013).
Chelation of iron ion (Fe2+)
The chelation effect test verifies the capacity of the molecule to inhibit the interaction of lipids and metals through the formation of insoluble metal complexes with iron ions. Iron ion chelation is an effective way to reduce and even eliminate the generation of hydroxyl radicals, since it prevents iron from interacting with H2O2, preventing its decomposition and the formation of more damaging free radicals (Melo et al., 2013).
To test whether the sample shows the action of chelating iron ion, the sample is usually dissolved in water, centrifuged, and has its supernatant mixed with ferrous hydrochloride and ferrozine. The absorbance reading is then taken at 560 nm and use is made of mathematical formulae to acquire the rate of the activity, such as the formula: {[Ablank − (Asample − Acontrol)] / Ablank} × 100 , in which Ablank, Asample and Acontrole are the absorbance of the blank, the sample and the control measured at 520 nm, respectively.
Iron ion chelating activity has already been tested for several species of brown algae, such as Ascophyllum nodosum (Tierney et al., 2013), Dictyota cervicornis (Costa et al., 2010), Dictyopteris delicatula (Costa et al., 2010; Florentin, 2015), Dictyopteris justii (Tierney et al., 2013), Dictyota menstrualis (Araújo, 2018; Costa et al., 2010), Dictyota mertensii (Costa et al., 2010), Fucus spiralis (Tierney et al., 2013), Fucus vesiculosus (Corsetto et al., 2020), Himanthalia elongata (Rajauria, 2019), Laminaria digitata (Rajauria, 2019), Laminaria saccharina (Rajauria, 2019), Lobophora variegata (Almeida, 2014), Pelvetia canaliculata (Tierney et al., 2013), Sargassum filipendula and Spatoglossum schroederi (Costa et al., 2010).
Other assays
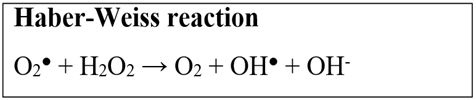
An assay also widely used to analyze the antioxidant activity of compounds is copper ion chelation. In biological systems, the balance of copper concentration is directly related to the regulation of cellular functions, causing several kinds of damage to the organism if homeostasis is broken (Yruela, 2005). When there is an increase in copper concentration, from the Fenton equation (Figure 1), and also due to the Haber-Weiss reaction (Figure 2), there is an increase in ROS production; therefore, with the chelation of copper ion, it is possible to observe the impediment of the formation of these. This analysis has already been observed in brown algae of the species Dictyota menstrualis (Araújo, 2018) and Dictyopteris justii (Melo et al., 2013), for example.
Fig. 2. Haber-Weiss reaction.
The cell antioxidant assay (CAA) is a method for quantifying antioxidant activity in cell culture (Wolfe & Rui, 2007). The principle of this assay is to pre-treat the cells with antioxidant compounds and dichlorofluorescin (DCFH-DA), a probe that penetrates the cells, after which a compound capable of generating free radicals is added, causing oxidation of the DCFH-DA into fluorescent dichlorofluorescein (DCF). The fluorescence emitted is measured by a fluorimeter. The lower the cellular fluorescence when compared to control cells, the higher the antioxidant capacity of the compounds (Wolfe & Rui, 2007). Corsetto et al. (2020) carried out the CAA with F. vesiculosus extract, using human hepatocarcinoma cells (HepG2), and obtained favourable results with 50-60% radical elimination, even with low concentrations of the actives.
Another in vitro assay used to evaluate the preventive capacity of brown seaweed extracts against free radicals is the evaluation of the enzymatic antioxidant activity. This technique consists of incubating the cells with the extract containing the seaweed's bioactive compounds and then adding a substance capable of generating oxidative stress from the generation of free radicals, after which the activity of the main antioxidant enzymes is measured (Berselli et al., 2010b). By analyzing the activity of catalase, glutathione reductase (GR) and glutathione peroxidase in HepG2 cells incubated with F. vesiculosus extract at a concentration of 62.5 µg/mL, Corsetto et al. (2020) observed that the extract had a protective effect on liver cells, as there was a significant reduction in GR and a small decrease in catalase activity, the latter not being statistically significant.
ANTIOXIDANT ACTIVITY IN VIVO
As highlighted, there are many algae that have compounds with antioxidant properties. However, many of these substances were only tested in vitro, and there are few reports in the literature using in vivo experiments. However, these studies demonstrate the importance of exploring the subject further, as they have great potential as pharmaceuticals and food improvers.
The pharmacological potential of the secondary metabolites of brown algae was demonstrated by Iwasaki et al. (2012) who, by adding fucoxanthin, extracted from Undaria pinnatifida, to the diet of obese and diabetic KK-Ay mice, observed a significant decrease in lipid hydroperoxide levels in liver and abdominal white adipose tissue (Iwasaki et al., 2012). Furthermore, fucoxanthin also decreased the level of glucose found in the blood of animals, a fact related to hyperglycemia, which has been shown to be induced by oxidative stress (Andrikopoulos, 2010). However, this antioxidant effect of scavenging reactive oxygen species (ROS) and/or free radicals was only found in diabetic animals, not appearing in normal mice.
In another similar study, but using an extract with phlorotanins and fucoxanthin from Undaria pinnatifida, Sargassum horneri and Cystoseira hakodatensis, a small lipid decrease in the liver was also observed, which was mainly related to the antioxidant activity of the fucoxanthin metabolites found in the organ (Airanthi et al., 2011).
Evaluating the antioxidant effect of phlorotanins and fucoxanthin, extracted from Fucus vesiculosus,Zaragozá et al. (2008) demonstrated that the administration of extracts with both substances in mice under normal conditions exhibited important antioxidant activity in vivo, eliminating oxygen and reducing active intermediates, which prevented the formation of oxidizing compounds, in addition to not presenting toxicity to the animal. In this study, it was also shown that extracts with lower amounts of phlorotannins (16.3 ± 0.3mg /100g) are more easily absorbed by the body than those with large amounts (27.7 ± 1.5mg /100g) (Zaragozá et al., 2008).
In addition to fucoxanthin and phlorotanins, in vivo studies with fucoidans are also found, such as the study by Kim et al. (2014), who extracted the polysaccharide from Ecklonia cava and tested its antioxidant activities against the oxidative stress induced by 2,2-azobis (2-amidinopropane) (AAPH) in a zebrafish model. In their results, Kim et al. (2014) obtained a higher survival rate in those individuals treated with AAPH and fucoidan extract than in those treated only with AAPH. The polysaccharide extract also showed significantly reduced levels of ROS production and lipid peroxidation (Kim et al., 2014).
In a more recent study by Wang et al. (2019), with fucoidan extracted from Sargassum fulvellum and also evaluating the antioxidant activity in zebrafish, the author's findings corroborate those of Kim et al. (2014), who suggested that the antioxidant activity of metabolites is related to the presence of the monosaccharides fucose, galactose and xylose. Furthermore, the results of this study, as well as those of Kim et al. (2014), showed suppression of ROS production and lipid peroxidation as well as cell death in AAPH-treated zebrafish. Furthermore, this study also demonstrated strong free radical scavenging activity by the sulfated polysaccharide (Wang et al., 2019).
In a different way, Moroney et al. (2015) used fucoidan and laminarin extracted from Laminaria digitata and added to the diet of pigs, to evaluate the possible contributions of these compounds on meat quality. It was observed that the antioxidant response reduced the amount of saturated fatty acids and lipid oxidation in the pork muscle, which in fact made the meat of better quality (Moroney et al., 2015).
Aiming to investigate the in vivo antioxidant and antibacterial activities of laminarin extracted from Cystoseira barbata, for the formulation of a topical cream with wound healing effect, Sellimi et al. (2018) observed that the use of laminarin helped in preventing free radical damage in living cells, which promoted better skin healing and regeneration in mice (Sellimi et al., 2018).
CONCLUSION
From this review, it is possible to conclude that there are several metabolites with antioxidant properties found in brown algae, as well as the differences and similarities between their extraction and isolation methods. The potential of this group of algae and its extracts for the pharmaceutical, food and cosmetic industries can also be seen, although more studies are needed to define the structure and the best method of extraction and isolation of each substance, as well as elucidating the mechanism of action, for its use in vitro and in vivo.