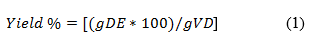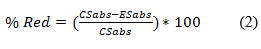Introduction
Cerrado domain is a Neotropical savannah, being this strategic environment with rich variability in species of Brazilian and world flora. In addition, it is considered the second largest in natural area, occupying an area of about 2 million km2, or 22 % of the National territory.1 In this environment a great diversity is found of plant families such as Malvaceae, subfamily Bombacoideae and Bombaceae tribe, which includes the Ceiba Miller genus, with about 17 species of Neotropical distribution. In Brasil 11 species of Ceiba are registered, Ceiba pubiflora being a species native to the Midwest and also to the Northeast and Southeast.2
C. pubiflora occurs mainly in semideciduous forests, and particularly in calcareous soils, and is popularly known as "potbellied or white paineira", with height between 15-25 m with canopy; gray stem with conical spines; compound leaves; fruits in capsule, oblong, woody, and brown seeds with ovoid shape. In addition to the Cerrado C. pubiflora is inserted in the flora of the Atlantic Forest and Caatinga biomes. In the Midwest region of Brasil the period of flowering and fruiting is associated by farmers during the rainy dry season. The flowers of C. pubiflora are large, pink color and have a light aroma which is why it is used in ornamentation and landscape projects in cities.3,4
Numerous extracts produced from the species of the Malvaceae family, present phytocompounds of great phytotherapeutic interest, for the food industries during conservation, in the pharmaceutical industry, in the production of topical and internal medicines, and in the agricultural industries such as larvicide, molluscicide and insecticide, and biotechnology mainly in the control of microorganisms.5-7
Genus Ceiba is described for various therapeutic, nutritional and economic purposes, in the form of floristic inventories and ethnobotanical studies based on the gum extracted from the stem bark and roots with aphrodisiac properties.4,8 However in relation to the phytochemical composition of the floral organ, little is known about the classes of phytocompounds and their possible phytotherapeutic effects requiring previous studies on phytochemistry that can be obtained from secondary metabolism.
Considering the scarcity of phytochemical studies and biological activities, this work aimed to evaluate the floral hydroethanolic extract of Ceiba pubiflora.
Material and methods
Plant material
Flowers were collected at the Instituto Federal Goiano, Rio Verde, Goiás State, Brasil (17°48′39.4″S and 50°53′57.5″W), at 8 pm on 25st March 2020. The plant material was identified and samples were deposited as voucher specimens in the Herbarium at the Instituto Federal Goiano, Rio Verde, Goiás State, Brasil (identification number HRV 12637).
Preparation of hydroethanolic extract
Extraction was carried out with 500 g flowers and 100 mL 70 % (v/v) hydroethanolic solution; it was kept under constant magnetic agitation for 12 h. Contact between solvent and raw material was kept for nine days at room temperature (25 °C), in the dark. It was manually agitated on a daily basis. The mixture that resulted from the extraction was separated by filtration (Unifil, C42), followed by solvent evaporation which was carried out by a rotary evaporator at reduced pressure. Then, the extract was lyophilized in a lyophilizer (Liotop, Mod. L101) with negative pressure until constant mass.
Phytochemical analysis
The phytochemical tests to detect the presence of cardiac glycosides, cyanogenic glycosides, alkaloids, organic acids, reducing sugars and no-reducing sugars, coumarins, foamy saponins and hemolytic saponins, polysaccharides, phenols, tannins, flavonoids, purines, resins, catechins, auronas and chalcones, depsides and depsidons, cyanogenic heterosides, benzoquinones, naphthoquinones and phenanthraquinones, anthraquinones, steroids and triterpenoids, sesquiterpenolactones, proteins and amino acids, leucoanthocyanidins, anthocyanins, azulenes, and oxidation time.9 These tests were based on the visual observation of color modification or precipitate formation after the addition of specific reagents. The results were expressed by the cross-testing (-) negative, (+) weak positive, (++) moderate positive and (+++) strong positive.
Physicochemical analysis
The yield of the dry extract was determined by calculating the ratio of its weight on a digital analytical scale (Marte, Mod. W220) with respect to the weight of the plant drug, expressed as a percentage according to equation (1).10
Where: DE = dry extraction, and VD = Vegetable drug.
The following chemical and physical-chemical parameters were performed as described by Valdés et al.11 and Domínguez et al.12 The pH was performed using a digital pH meter (Lucadena, Mod. 210-P) (IAL, 2008), the total solids content in an oven at 105 °C (Nova Ethics, Mod. 400-3ND) (IAL, 2008), and the refractive index in a digital refractometer (Hanna Instruments, Mod. HI96800) with a refraction range between 1,333 0 and 1,508 0 nD. The relative density was determined in a 10 mL graduated cylinder (Pyrex), and expressed in g mL-1 at 20 °C. Chromatography determination was performed by thin-layer chromatography (TLC) for the extract using chromatoplates (DC-Fertigfolien Alugram® Xtra SIL G/UV254) (Macherey-Nagel). Approximately 10 µL of the extract was deposited on the chromatoplates cut into strips of (2 x 10 cm in length). The eluents used were (ethyl alcohol, chloroform, ethyl acetate and acetone), and the following developers used (sulfuric vanillin, ultraviolet light, long and short wavelengths, and ferric chloride) were used. The retention factor (Rf) was determined according to the points observed for each developer.13 The hydroethanolic extract was scanned between 250 and 900 nm in a UV-Vis spectrophotometer (Belphotonics, Mod. M-51), the data were generated by UV-Vis professional 2 software and processed in Origin.
Antioxidant activity and total phenolic compounds
The antioxidant capacity was determined using the DPPH (2,2-diphenyl-1-picrilhydrazyl) reduction method. The DPPH sequestration method was performed in microdilution in 96-well microplates (Global Plast) modified.14 For each well, 100 µL of an ethanolic solution with DPPH at the concentration of 0,06 mMol L-1 and 100 µL of a hydroethanolic extract solution at different concentrations. The microplate was kept in a place protected from light and heat for 1 h. Then, the microplate spectrophotometer (Scientific Hexis, SpectraMax Plus, Mod. 384) was read at a wavelength of 517 nm. The percentage of antioxidant capacity was determined according to equation (2). The inhibition concentration (CI50) was determined in (mg L-1) in the sequestration of 50 % of the initial concentration of the standard solution of DPPH 0.06mMol L-1.
Where: CSabs = absorption of the control solution, and ESabs = absorption of the extract solution containing the radical DPPH.
In order to quantify total phenolic compounds, 1,9 mL Folin-Ciocalteu reagent in distilled water (1:10, v/v) was added to 200 μL extract hydroethanolic floral. To neutralize the mixture, 1,9 mL aqueous solution of sodium carbonate (60 g L-1) was used. The reaction was kept in the dark at room temperature for 120 min. Then, absorbance was measured at 725 nm. Calculation was carried out with the use of the standard curve and results were GAE expressed as mg gallic acid 100 g-1 dry extract (DE).13
Results and Discussion
C. pubiflora floral period in the collection area in the municipality of Rio Verde, Goiás State, Brasil in 2020 was between the months of March to May, with a high intensity of flowering in May. The (figure 1) shows a flower of C. pubiflora used in the production of hydroethanolic floral extract of this study.

Fig. 1 Ceiba pubiflora flower used in the hydroethanolic floral extract, collected in Rio Verde, Goiás State, Brasil in 2020. Bar: 10 cm. Source: Authors, 2020
C. pubiflora extract floral presented transparency, crystallinity, pink color, and light aroma. The percentage of dry extract, yield equal to 6,88 % (w/w). The phytochemical prospection indicated the presence of different classes of secondary metabolites, except foam saponins, polysaccharides, resins, benzoquinones, anthraquinones, proteins and amino acids. Table 1 shows the results of the phytochemical screening carried out on the hydroethanolic floral extract.
Table 1 Qualitative phytochemical prospection of the floral hydroethanolic extract of Ceiba pubiflora
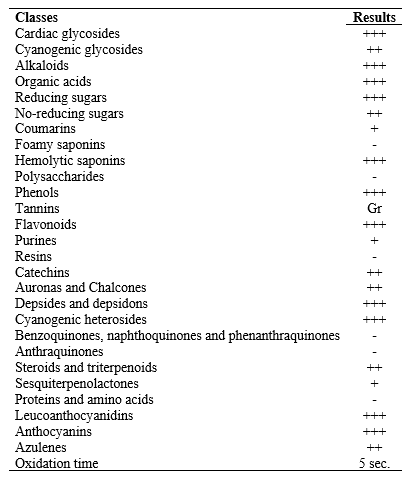
Note: (-) negative. (+) weak positive. (++) moderate positive. (+++) strong positive. Tannins: Green (Gr) condensed or catechic. Blue (Bl) hydrolyzable or gallic. Source: Authors, 2020.
The extract yield was high, although it is not possible to compare it with other floral extracts of the Ceiba genus due to lack of study. Through qualitative phytochemical prospection of the floral extract of C. pubiflora, it was possible to determine the presence of special metabolites classes that present in several studies a wide variety of biological activities such as antimicrobial, antioxidant, antitumor, antiophidic, photoprotective and photosensitizing.15,16
Tannins and flavonoids have antimicrobial properties, 17 saponins have cytotoxic action 18 and depsides and depsidons have anti-inflammatory properties.19 Although some reagents may reveal the presence of more than one phytoclass, this is only a preliminary result, requiring further testing using quantitative results that can be obtained by high performance liquid chromatography and compared to classical standards. The expressive content of phenolics in the floral extract of C. pubiflora, as well as in several other studies, has reported important biological actions. According to Oliveira et al.20 Haslam, 21 and Dixon et al.22) phenolic compounds inhibit lipid peroxidation and lipoxygenase in vitro. The oxidation time was slightly fast, suggesting that it is a potent extract with antioxidant characteristics.
Qualitative hemolytic assay on human erythrocytes, demonstrated potential cytotoxicity with a minimum of 5 min, and complete hemolysis with 10 min. After five minutes, deformation of the human erythrocytes can be observed, followed by rupture and worsening of hemoglobin. Figure 2 shows in the hemolysis assay on human erythrocytes from the floral extract of C. pubiflora.
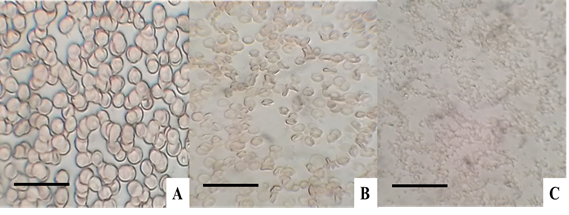
Fig. 2 Hemolysis assay in 5 % blood cells concentrate. In (A) normocytic blood cells, in (B) hemolysis with 5 min, and (C) hemolysis with 10 min. Bars: (A) 150 µm, (B) 100 µm and (C) 100 µm. Source: authors, 2020
The pH was 6,41 ± 0,02, total solids of 1,12 ± 0,09, refractive index of 1,536 9 ± 0,13, and relative density of 0,906 1 ± 0,01 g mL-1 to 20 °C. According to Menezes Filho et al., 13 the acid or alkaline pH in extracts, may be related to the classes of phytocompounds of a phenolic or saponinic nature. The content of total soluble solids varies according to the plant extracts and types of solvents used. Soluble solids represent the volatile organic and inorganic material content of the extract solution.
Acetone and ethyl acetate were the eluents that had the highest number of Rf (12), respectively. The presence of sugars reducing, glucose and fructose, and no-reducing sucrose was observed by thin-layer chromatography analysis in the floral extract of C. pubiflora (table 2).
Table 2 Results of thin-layer chromatography of the floral extract of Ceiba pubiflora
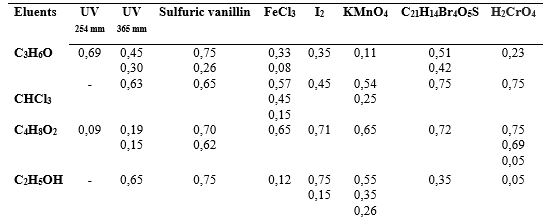
Note: Results in Rf (Retention factor), determined in millimeters (mm). Source: Authors, 2020
The chromatographic assay, a large amount of Rf is observed for the four eluents evaluated and their respective developers. Only polar eluents were used, observing that the compounds of the flower extract showed to be less polar and were more easily eluted. The elotropic sequence of the eluents was: CHCl3 (10) Rf > C4H8O2 (12) Rf > C3H6O (12) Rf > C2H5OH (10) Rf, the Rf showed high values in mm, which confirms that they have a greater amount of apolar compounds in the extract. Especially acetone and ethyl acetate proved to be the best eluents.
The developers UV254 nm light shows phytochemicals that absorb light, often they are conjugated substances and aromatic systems and for UV365 nm light the compounds naturally fluoresce; sulfuric vanillin is especially sensitive to the presence of alcohols and terpenoids; the ferric chloride complex with phenols and enolizable compounds; complex I2 with structures of amino acids, indoles, alkaloids, steroids, purines and lipids; potassium permanganate easily stains oxidizable compounds, olefins, alkynes and aromatics; bromocresol green shows organic acid compounds; and chromic acid difficult substances.23,24
The results obtained for both qualitative tests and for TLC showed that both are suitable for a preliminary analysis of phytochemicals, presenting speed, economy, safety and easy handling.24) The presence of reducing sugars was more intense both in the qualitative test (table 1), and in the TLC, with two reducing sugars (glucose and fructose) and one non-reducing sugar (sucrose). Table 2 was the results of the thin-layer chromatography of the floral extract of C. pubiflora in different eluents.
The UV-Vis spectrophotometric analysis, between 850-250 nm short and intense bands were observed, suggesting the presence of phenolic and flavonoid compounds that absorb in UV energy sources between 200-300 nm and between 400-500 nm, respectively.25 Figure 3 shows the UV-Vis spectrophotometry scan of the floral hydroethanol extract of C. pubiflora between 850-250 nm.
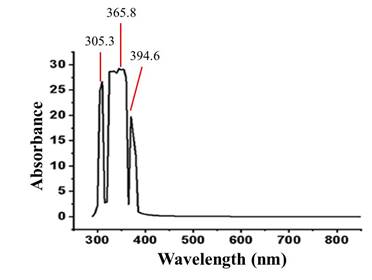
Fig. 3 UV-Vis spectrophotometry of the Ceiba pubiflora floral hydroethanolic extract between 850-250 nm. Source: Authors, 2020
The antioxidant activity of the floral extract of C. pubiflora presented IC50 = 217,4 ± 0,19 mg L-1, and total phenols content of 7,26 ± 0,16mg GAE 100g-1 in (DE). Comparing the phytochemical qualitative and quantitative methods, it is observed that in (table 1), the groups of phenolics, tannins and flavonoids evaluated by colorimetric and precipitation methods showed superior results by the cross test, when compared to the quantitative tests in the radical reduction DPPH and total phenolic content, where the first is visually observed and the second more refined, is performed through standard curves. According to Menezes Filho et al., 13 Degáspari and Waszczynsyj 26 and Camacho-Campos et al. 27 antioxidants, and total phenols content are complex groups found mainly in vegetables that have functions such as delaying or inhibiting the oxidation of lipids or other biomolecules, thus preventing the onset of oxidation chain reactions.
Conclusions
The floral extract of Ceiba pubiflora exhibited in this study, a great diversity of phytochemical groups present in the qualitative methodology. In addition, the extract showed potential antioxidant activity and expressive content of total phenolic compounds. Future studies should be carried out evaluating the phytochemical profile by high performance liquid chromatography, other antioxidant models and other biological activities.