Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Estomatología
versión impresa ISSN 0034-7507versión On-line ISSN 1561-297X
Rev Cubana Estomatol vol.55 no.1 Ciudad de La Habana ene.-mar. 2018
ARTÍCULO DE REVISIÓN
Alternatives of surface treatments for adhesion of lithium disilicate ceramics
Alternativas de tratamientos de superficie para adhesión de cerámica de disilicado de litio
Alberto Carlos-Cruz González,I Edgar-Delgado MejíaII
I Department of Oral Health, Faculty of Dentistry, Universidad Nacional de Colombia. Bogotá, Colombia.
II Department of Chemistry, Faculty of Sciences, Universidad Nacional de Colombia. Bogotá, Colombia.
ABSTRACT
Introduction: the clinical success of a restoration is strongly associated with the quality and durability of the ceramic-cement resin interface. In order to obtain an adequate union between these materials of different nature surface treatments are used and achieve mechanical retention or chemical interaction.
Objectives: to check if any method promotes a true chemical bond between lithium disilicate ceramics and resin cement. As well as determineif there is any treatment that reports bonding values comparable to hydrofluoric acid and silane (gold standard).
Methods: a systematic literature review was developed based on the PRISMA strategy, where the databases were searched: Science Direct, Pubmed (MEDLINE), EMBASE, Springer Journal, SciELO with MeSH and free terms from 2005 to November 2016 for articles in English and Spanish on surface treatments for lithium disilicate.
Results: from 58 publications selected a sample of 21 articles. Two articles reported high risk of bias.
Conclusions: hydrofluoric acid and silane continue to be the method with the highest and most reliable adhesion values in the literature. Universal adhesives are an alternative to promote chemical adhesion additional to the silane. Diamond burs, Nd: YAG and Er: YAG laser are not recommended as surface treatments.
Keywords: glass ceramics; IPS e.max Press; IPS e.max CAD; hydrofluoric acid; lithium-disilicate-glass ceramic; surface treatment; silane; universal adhesive.
RESUMEN
Introducción: el éxito clínico de una restauración se asocia fuertemente a la calidad y duración de la interface cerámica-cemento resinoso. Para que exista una adecuada unión entre estos materiales de distinta naturaleza se emplean tratamientos de superficie para lograr una buena retención mecánica o interacción química.
Objetivos: revisar si algún método promueve una verdadera adhesión química entre la cerámica de disilicato de litio y el cemento resinoso, así como determinar si existe algún tratamiento que reporte valores de unión comparables al ácido fluorhídrico y silano (patrón de oro).
Métodos: se desarrolló una revisión sistemática de literatura basada en la estrategia PRISMA, donde se buscó en las bases de datos: Science Direct, Pubmed (MEDLINE), EMBASE, Springer Journal, SciELO con términos MeSH y libres desde el 2005 a noviembre de 2016 para artículos en inglés y español sobre tratamientos de superficie para disilicato de litio.
Resultados: de 58 publicaciones, se seleccionó una muestra de 21 artículos. Dos artículos reportaron riesgo de sesgo alto.
Conclusiones: el ácido fluorhídrico y silano continúan siendo el método con los valores de adhesión más altos y confiables de la literatura. Los adhesivos universales son una alternativa para promover adhesión química adicional al silano. Fresas diamantadas, laser Nd: YAG y Er:YAG no se recomienda como tratamientos de superficie.
Palabras clave: cerámica vítrea; IPS e.max Press; IPS e.max CAD; ácido fluorhídrico; cerámica vítrea de disilicato de litio; tratamiento de superficie; silano; adhesivo universal.
INTRODUCTION
There is a growing demand for the use of all-ceramic restorations by dentists and patients, in order to satisfy high needs as esthetic, biocompatibility and longevity.1 The lithium disilicate (Li 2Si2O5) is a glassy ceramic with a flexural strength average of 400 MPa and a favorable translucency, indicating its to use in anterior and posterior sector.2 This material is recommended for inlays, veneers and anterior or posterior crowns supported by teeth or implants.3 The lithium disilicate system, IPS e.maxTM, reports a survival rate of 97.4 % and 94.8 % for five and nine years of use, respectively, in anterior and posterior crowns.4 For fixed prostheses of three units, survival and success similar to metal-ceramic systems is reported to 10-year old, with catastrophic failure only in molar teeth.5 However, the clinical success of a ceramic restoration does not only depend on the intrinsic properties of the material, this is strongly associated with the quality and duration of the resin cement-ceramic interface.6 In order for there to be an adequate bond between two materials of different nature, organic (resin cement) and inorganic (ceramic), a conditioning is necessary to increase the surface energy of the ceramic, and to improve its bonding to the cementing agent, either by mechanical retention or chemical reaction.7
The etching with hydrofluoric acid followed by silanization is considered as the gold standard surface treatment for vitreous ceramics.8 Hydrofluoric acid, in concentrations between 4.6 and 9.6 %, creates roughness on the surface by dissolving part of the glass matrix, while the silane agent acts as a bifunctional molecule with an organic and inorganic termination to promote chemical bonds.9 However, this etching is considered some controversial, as it is done with a highly corrosive inorganic acid, which is a potential risk for those who manipulate.10 In addition, hydrofluoric acid may have a negative influence on the flexural strength of the lithium disilicate, which decreases over time in contact and concentration of the acid.11 Although, there are some reports of the reinforcement of the mechanical properties once the cementing agent is applied to the etched surface.12,13
The objective of this review was to check if any method promotes a true chemical bond between lithium disilicate ceramics and resin cement, as well as to determine if among the different methods proposed, there is one that reports bond values comparable to those obtained with hydrofluoric acid and silane, currently considered as the gold standard method. The review question was defined as: what methods exist in the literature that promote adhesion by chemical and/or physical phenomena similar or superior to the gold standard, defined as etching with hydrofluoric acid and silane?
METHODOLOGY
A systematic literature review was developed based on the PRISMA strategy adapted forPereira et al. en el 2016.14,15 According to the PICOs strategy, the parameters in this review were lithium disilicate ceramics, IPS e.max Press or CAD as the population, surface treatments to define the intervention, without treatment ceramic or surface treatment gold standard, etching with hydrofluoric acid and silane as comparison, increase in adhesive or bond strength values as results and in vitro experimental studies to define study design.
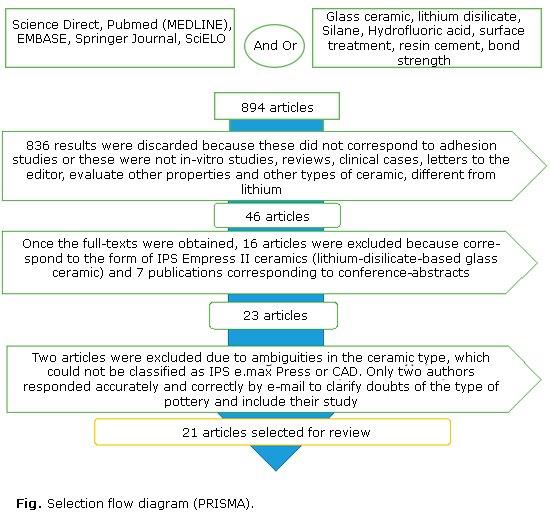
An electronic search was carried out in the databases Science Direct, Pubmed (MEDLINE), EMBASE, Springer Journal, Scielowith the following MeSH and free terms: glass ceramic, lithium disilicate, silane, hydrofluoric acid, surface treatment, resin cement, bond strength by combining with the Boolean connectors AND and OR (Fig.). For the Springer Journal database, "all words" were used to replace AND, "at least one word" for OR and the exact phrase for quotation marks ("surface treatment").The search combinations were as follows: glass ceramic AND silane OR hydrofluoric acid OR "surface treatment" AND bond strength; lithium disilicate AND silane OR hydrofluoric acid OR "surface treatment" AND bond strength; glass ceramic AND "surface treatment" AND bond strength; lithium disilicate AND "surface treatment" AND bond strength. For example in Em base the results added up to 644, 784, 39 and 8 with each search combination respectively. The cross-repeats were eliminated in the search formulas and with the other databases, in order to finally select five possible publications of Embase. A second example was the search in SciELO, where we obtained 4, 1, 4, 1 publications respectively. Finally it filtered to have only three possible publications to select.
Inclusion criteria were defined as articles from experimental studies that evaluated the effect of surface treatments on the bond strength of lithium disilicate ceramics (IPS e.max Press and / or IPS e.max CAD), published in a period between 2005 and November 2016, in English or Spanish. In addition, for inclusion in the sample the publications should have a clear and reproducible methodology, a quantitative measurement of the results, an inter-group comparative statistical analysis. In contrast, clinical studies, literature reviews, clinical cases and letters to the editor were excluded. In addition, publications with evaluation of only IPS Empress II lithium disilicate ceramic or other form of disilicate than those specified in this paper were excluded, publications in different period of established range, confusing and non-specific methodologies, and studies with results that did not report values bond strength. The selection and evaluation process of all-articles was carried out by the two authors (A.C.C.G. and E.D.M.), but if there were differences between evaluations, a third evaluator as a guest would be used.
For the evaluation of the risk of bias of the publications, these were submitted to an instrument that should answer the following questions: Was the size of the sample considered representative?, randomization of ceramic samples?, ceramic sintering according to manufacturer's specifications?, were the adhesive resistance tests performed by an operator without risk of bias (blind)? the reproducible methodology?, was there a positive and/or negative control group?, test executed following International Standard Rules, as ISO, ASTM or other? The score for assessing these questions was 0 to 2, where 0 if the parameter was clearly reported;1 if the parameter was mentioned, but the precision of the execution was not clear and 2 the parameter was not mentioned in the document or the information was not present. Finally, the risk was categorized into: 0-4 for low risk (L), 5-9 for medium risk (M), 10-14 for high risk (H).14 In the question of norms for execution of tests, if the information was not observed in the study, but the execution parameters correspond to the stipulated by the norms of adhesion ISO were assigned value of 1 and not 2. If any author answered any questions about the information in his study that would allow him to continue his selection, the qualification of this study for risk of bias was made considering that such missing or confusing information was not present (score 2).
RESULTS
The results of the search determined 58 publications to review the full text, of which 21 articles were finally selected for analysis in the review. The selection process is described in figure. The characteristics of the information are described in table 1.
The results of risk of bias reported two articles with high risk and the other 19 publications presented an average risk. Only two publications (A10 and A21) were classified as not reproducible because, although the author responded correctly on the type of ceramic used and diameter dimensions of the adhesive area, the single information within the article was not clear to identify those parameters. No information was found on the knowledge or status of the operator of the tests performed (blind operator). The results of risk of bias are described in table 2.
DISCUSSION
According to the information obtained for this review, there are constant investigations of alternatives for surface treatments or methods that optimize adhesion values, even by modifying the established gold standard.In all the studies of the sample, the etching with hydrofluoric acid followed by silane agent was evaluated, which allowed to corroborate the affirmation that this association is considered as the positive control group or gold standard in those investigations.
HYDROFLUORIC ACID
Hydrofluoric acid etching followed by silane reports the best adhesive strength values compared to other methods, such as sandblasting, lasers and roughening with diamond bur.16-20 Another alternative of etched agent for ceramics is titanium tetrafluoride. It reported bond strength values similar to hydrofluoric acid, after a considerable laboratory aging were observed in these samples debonded spontaneously, because of this it does not yet allow to recommend the titanium tetrafluoride as a reasonable substitute.21
Hydrofluoric acid etching is a protocol-sensitive method, with concentration and time of use as variables that play a crucial role in bond strength values. The concentrations currently available in the market are approximately 4.6 and 9.6 % and these get to their best performance of bond strength in lithium disilicate between 20 and 60 s of etching.22 Hydrofluoric acid concentrations influence adhesion, such as assessed Sundfeld et al.23 in 2015 where they recorded for 20 s in concentrations of 1.5, 2.5, 5, 7.5, 10 and 15 %, determining that 7.5 % presented better statistically significant values in relation to the first three groups and without significant differences with higher concentrations. The first three groups were statistically equal to each other.A second study by the same authors evaluated the same model of the first five concentrations of acid, but they added the temperature variable, heating the acid to 70 °C during etching or ceramic at 85 °C with hot-air or a combination of both. It was observed that the heat treatment in all its forms improved the adhesion values for the first three groups, as for the groups of 7.5 and 10 % applying the temperature methods separately had better result than when applied in combination, values of the latter, which was even lower than the control groups.24 This opens the possibility of decreasing concentrations of the etching agent by maintaining the levels of adhesion offered by this method, however these variables require more research and evaluation in the long term to formulate comparable results.
SILANE
On the other hand is the silane coupling agents, it is preceded by a mechanical-retention method such as hydrofluoric acid,the silane proved to increase the values of bond strength, compared to the single action of roughness or mechanical-retention.25 A modification in the silanization protocol includesthe elevation of temperature of the silane when drying it with hot air17,26,27 or in some cases washed with hot water,28,29 it in order to obtain an optimize the adhesive results. According to the increase in temperature, drying at 45 °C associated with an non-functional silane does not appear to significantly increase bond strength values.17 Abduljabbar et al. 26 reported that a functional silane subsequent to etching with hydrofluoric acid increases the adhesion values as compared to the etch alone, further the drying at 100 °C for 5 min significantly improves the results compared to the two previous groups. According to the limited information obtained, the drying at temperatures between 45 to 100 °C of silane agents can improve on average between 2 and 3 MPa the values of adhesive strength in lithium disilicate, compared to drying at room temperature.17,26 Meanwhile Yavuz et al.27 tested temperatures of 60 and 100 °C in two commercial functional silane systems, where they reported higher differences (between about 4.7 and 6.8 MPa) of adhesive strength compared to drying at room temperature. However, no differences were observed between both tested temperatures, but there were commercial silane houses with this protocol. All of the above may mean that the drying at higher temperatures of both functional and non-functional silanes, is not a procedure as indispensable as the use of silane itself after the acid etching in the dental ceramics analyzed in this review. But it is clear that it can increase the adhesive bond strength.
For the second modification of the silane protocol, washing with distilled water at 80 °C for 15 or 30 s after application of silane, this proved to be a procedure without significant influence for the adhesion as compared to the drying with hot air.28,29 Therefore, when it is decided to include a protocol with temperature associated with the silane, it is sufficient to dry with hot air and it is not necessary to add the washing with hot water to the protocol.29 Elevating the temperature of the silane, with hot air, it is conducted in order to remove by-products and the vehicle (acetic acid, water and alcohol) in order to make the reaction efficient, promoting the initiation of siloxanes.27,29 On the other hand, washing with hot water goes towards decreasing the layer thickness, eliminating external layers of the silane that are covalently bonded together, and this allows the reaction of the layer most strongly chemically-bonded to the ceramic surface.29
UNIVERSAL ADHESIVES AND MULTIPURPOSE ADHESIVES
Another relatively recently studied chemical alternative is multimodal or multipurpose adhesives and universal adhesives (table 3). Universal adhesives are simplified adhesives in a single bottle, suitable for different substrates such as dentin, enamel, resins, alloys and ceramics.30 Universal adhesives contain silane and phosphate monomers called 10-methacryloxydecyl dihydrogen phosphate (MDP), these monomers are responsible for the adhesive capacity of these adhesives between the ceramic, polymeric and dental substrates.31 On the other hand, the multipurpose adhesives are systems with option of dual-polymerization, or only chemical that are available in presentations of two bottles usually, indicated in different clinical protocols, specifically when photopolymerization is not an option.
The use of adhesive systems subsequent to the silane agent in the adhesion protocols, mainly in ceramic-repair with composite resin,this to improve the penetration of adhesive molecules into the ceramic irregularities.23,32 Sundfeld et al.23 evaluated the association of anon-functional silane with a multipurpose or dual-cured adhesive, this process preceded by different concentrations of hydrofluoric acid, and demonstrated a significant increase in bond strength compared to silane alone. In addition they reported a better infiltration of irregularities with this combination.In contrast, in another study there was no increase in the bond strength values of three resin cementswith functional silane followed by a dual-cured or multipurpose adhesive compared to the same silane alone.33 Probably these contradictory results are due to the type of silane that was used in each study, the non-functional silane is mainly composed of ethyl alcohol, water and methacryloxypropyl trimethoxysilane,30 while the functional silane is composed of ethanol, 3-trimethoxysilylpropyl methacrylate, phosphoric acid methacrylate ester (MDP) and disulfide acrylate. 33 The latter components suggest a higher layer thickness added to the adhesive, where it is expected to find dimethacrylates, HEMA, and phosphoric acid acrylate among other components (table 3).
It is clear from the revised information that the use of silane is an essential process following a mechanical action method and can not be replaced by a universal or multipurpose adhesive.30,31,34 However, when it is decided to use universal or multipurpose adhesive in the adhesion protocol, it may be more convenient to use a non-functional silane,30,31 and not resort to the synergy bewteen activated silanes and universal or multipurpose adhesives.
AIR-PARTICLE ABRASION OR SANDBLASTING
It´s clear in the literature that the bond strength values reported by sandblasting with particles of aluminum oxide are inferior when compared to etching, with or without the use of silane.16-18,32,35 However, Guarda et al.32 reported values of bond strenght comparable to that etched-hydrofluoric acid, after subjecting two study groups to 3 000 thermal cycling and 100 000 fatigue cycles. In contrast, another report suggests very low values compared to acid etching to only 1 000 cycles.35
In summary, sandblasting is a surface treatment that fails to obtain union strength values similar to acid etching, but is an option available when there is no access to hydrofluoric acid. On the other hand, it is convenient to analyze the effects of this method on the properties of lithium disilicate, but this was not an objective in this review.
DIAMOND BURS
There are other surface treatments proposed in the literature, such as diamond burs, tribological treatment with cojet TM and lasers. 16,19,20,34,35 In particular the use of medium-grain diamond burs (30 μm grain size), such as red halo, can produce roughness values comparable to those of hydrofluoric acid but it does not provide sufficient bond strength values to be an alternative to acid etching.19,20 Using these burs and silane agents or universal adhesives does not improve adhesion.34
TRIBOCHEMICAL SILICA COATING
For the cojetTM or sandblasted with aluminum oxide particles (30 μm) coated with silica, only one of the articles that evaluated this method19,20,34, reported values comparable to those obtained with etching with hydrofluoric acid (literature review code A10).35 With these contradictory results it is advisable to obtain more publications with comparable methodologies before establishing a reliable recommendation.In addition, this treatment was evaluated under a macro-shear bond strength test, it would be useful to execute micro-shear or micro-tension bond-strength test to corroborate results. Therefore, tribochemical silica coating, in the opinion of the authors in this review, can not yet be recommended with an efficient alternative to etching and silanizing.
LASERS
The lasers reported in this article include the Nd:YAG,16 the Er:YAG19 and a femtosecond laser, consisting of a titanium system: sapphire oscillator.35 The systems doped with neodymium and with erbium are used with the aim of increasing the roughness of the ceramic material. The third system found refers to an ultra-short pulse laser, used in medicine and the materials industry, in order to cause ablation on the surface in a precise and reproducible way, without the thermal collateral effects on the ceramics.35 The Nd: YAG and Er: YAG systems, with the limitations of the evaluated parameters of power, duration, energy density, among others, did not show significant results in comparison with the etching with hydrofluoric acid.16,19 However, when comparing the three laser systems with each other, the femtosecond laser is considered to be significantly superior, offering almost double bond strength values.35 But when comparing these, with the cojetTM system, a significant superiority of the cojetTM compared to the three laser systems.19,35
CONCLUSIONS
With the limitations and heterogeneities of all the information previously analyzed, it can be concluded that etching with hydrofluoric acid and silane, continues to be the method with the highest bond strength values and reliable over time, according to the literature. However, the modification in the etching and silanization protocols can achieve optimization of bond strength results.
The use of universal and multipurpose adhesives is a useful alternative to promote chemical adhesion in lithium disilicate,mainly at the time of a ceramic repair with composite resin. However, the only molecules responsible for promoting true chemical adhesion to lithium disilicate are silanes and phosphate monomers (MDP).
The use of the cojetTM and the femtosecond laser demonstrate possible future alternatives, however, these require more research in order to establish a recommendation. The mechanical retention by diamond burs, the Nd: YAG laser and the Er: YAG laser are not recommended as surface treatments in lithium disilicate ceramics.
Conflict of interest
No conflict of interest exists among the participants in the study.
BIBLIOGRAPHIC REFERENCES
1. Guess PC, Schultheis S, Bonfante E, Coelho PG, Ferencz JL, Silva NRF. All-ceramic systems: laboratory and clinical performance. Dent Clin North Am. 2011 [cited 2016 Sep 2];55(2):333-52,ix. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21473997
2. El-Meliegy E, van Noort R. Lithium Disilicate Glass Ceramics. In: Glasses and Glass Ceramics for Medical Applications SE - 12. New York: Springer; 2012. p. 209-18 [cited 2015 Sep 22]. Available from: http://dx.doi.org/10.1007/978-1-4614-1228-1_12
3. Li RWK, Chow TW, Matinlinna JP. Ceramic dental biomaterials and CAD/CAM technology: State of the art. J Prosthodont Res. 2014 Aug 26 [cited 2015 Sep 22];58(4):208-16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25172234
4. Gehrt M, Wolfart S, Rafai N, Reich S, Edelhoff D. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin Oral Investig. 2013;17(1):275-84.
5. Kern M, Sasse M, Wolfart S. Ten-year outcome of three-unit fixed dental prostheses made from monolithic lithium disilicate ceramic. J Am Dent Assoc. 2012 [cited 2015 Sep 22];143(3):234-40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22383203
6. Della Bona A, Borba M, Benetti P, Pecho O, Alessandretti R, Mosele J, et al. Adhesion to Dental Ceramics. Curr Oral Heal Reports. 2014 [cited 2015 Oct 12];1(4):232-8. Available from: http://dx.doi.org/10.1007/s40496-014-0030-y
7. Lung CYK, Matinlinna JP. Aspects of silane coupling agents and surface conditioning in dentistry: an overview. Dent Mater. 2012 [cited 2015 Oct 12];28(5):467-77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22425571
8. Tian T, Tsoi JKH, Matinlinna JP, Burrow MF. Aspects of bonding between resin luting cements and glass ceramic materials. Dent Mater. 2014 [cited 2015 Sep 22];30(7):e147-62. Available from: http://dx.doi.org/10.1016/j.dental.2014.01.017
9. Ho GW, Matinlinna JP. Insights on Ceramics as Dental Materials. Part II: Chemical Surface Treatments. Silicon. 2011 [cited 2015 Sep 22];3(3):117-23. Available from: http://link.springer.com/10.1007/s12633-011-9079-6
10. Ozcan M, Allahbeickaraghi A, Dündar M. Possible hazardous effects of hydrofluoric acid and recommendations for treatment approach: a review. Clin Oral Investig. 2012;16(1):15-23.
11. Zogheib LV, Bona AD, Kimpara ET, McCabe JF. Effect of Hydrofluoric Acid Etching Duration on the Roughness and Flexural Strength of a Lithium Disilicate-Based Glass Ceramic. Braz Dent J. 2011;22(1):45-50.
12. Sato P, Cotes C, Yamamoto LT, Rossi NR, Macedo C. Flexural strength of a pressable lithium disilicate ceramic : influence of surface treatments. Appl Adhes Sci. 2013;1:3-7.
13. Xiaoping L, Dongfeng R, Silikas N. Effect of etching time and resin bond on the flexural strength of IPS e.max Press glass ceramic. Dent Mater. 2014 [cited 2015 Sep 2];1-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25189110
14. Pereira GKR, Fraga S, Montagner AF, Soares FZM, Kleverlaan CJ, Valandro LF. The effect of grinding on the mechanical behavior of Y-TZP ceramics: A systematic review and meta-analyses. J Mech Behav Biomed Mater. 2016 [cited 2015 Sep 2];63:417-42. Available from: http://dx.doi.org/10.1016/j.jmbbm.2016.06.028
15. Pereira GKR, Venturini AB, Silvestri T, Dapieve KS, Montagner AF, Soares FZM, et al. Low-temperature degradation of Y-TZP ceramics: A systematic review and meta-analysis. J Mech Behav Biomed Mater. 2016 [cited 2015 Oct 12];55:151-63. Available from: http://dx.doi.org/10.1016/j.jmbbm.2015.10.017
16. Yucel MT, Aykent F, Akman S, Yondem I. Effect of surface treatment methods on the shear bond strength between resin cement and all-ceramic core materials. J Non Cryst Solids. 2012;358(5):925-30.
17. Colares RCR, Neri JR, de Souza AMB, Pontes KMDF, Mendonça JS, Santiago SL. Effect of surface pretreatments on the microtensile bond strength of lithium-disilicate ceramic repaired with composite resin. Braz Dent J. 2013;24(4):349-52.
18. Aboushelib MN, Sleem D. Microtensile bond strength of lithium disilicate ceramics to resin adhesives. J Adhes Dent. 2014 [cited 2015 Oct 12];16(6):547-52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25516886
19. Erdemir U, Sancakli HS, Sancakli E, Eren MM, Ozel S, Yucel T, et al. Shear bond strength of a new self-adhering flowable composite resin for lithium disilicate- reinforced CAD/CAM ceramic material. J Adv Prosthodont. 2014 [cited 2015 Sep 22];6:434-43. Available from: http://dx.doi.org/10.4047/jap.2014.6.6.434%5Cnhttp://jap.or.kr
20. Neis CA, Albuquerque NLG, de Souza Albuquerque I, Gomes EA, de Souza-Filho CB, Feitosa VP, et al. Surface treatments for repair of feldspathic, leucite- and lithium disilicate-reinforced glass ceramics using composite resin. Braz Dent J. 2015;26(2):152-5.
21. Klosa K, Boesch I, Kern M. Long-term bond of glass ceramic and resin cement: evaluation of titanium tetrafluoride as an alternative etching agent for lithium disilicate ceramics. J Adhes Dent. 2013;15(4):377-83.
22. Pérez CC, Correa FL, Janeth LUZ, Hoyos A, Gaviria G. In vitro evaluation of the effect of hydrofluoric acid concentration and application time on adhesion to lithium disilicate. Rev Fac Odontol Univ Antioq. 2014 [cited 2015 Sep 22];26(1):62-75. Available from: http://ref.scielo.org/3kbhxx
23. Sundfeld Neto D, Naves LZ, Costa AR, Correr AB, Consani S, Borges GA, et al. The Effect of Hydrofluoric Acid Concentration on the Bond Strength and Morphology of the Surface and Interface of Glass Ceramics to a Resin Cement. Oper Dent. 2015;40(5):470-9.
24. Sundfeld D, Correr-Sobrinho L, Pini NIP, Costa AR, Sundfeld RH, Pfeifer CS, et al. Heat treatment-improved bond strength of resin cement to lithium disilicate dental glass-ceramic. Ceram Int. 2016 [cited 2015 Sep 2];42(8):10071-8. Available from: http://dx.doi.org/10.1016/j.ceramint.2016.03.112
25. Gré C, de Ré Silveira R, Shibata S, Lago C, Vieira L. Effect of Silanization on Microtensile Bond Strength of Different Resin Cements to a Lithium Disilicate Glass Ceramic. J Contemp Dent Pract. 2016;17(2):149-53.
26. Abduljabbar T, Al-Qahtani MA, Jeaidi ZA, Vohra F. Influence of silane and heated silane on the bond strength of lithium disilicate ceramics - An in vitro study. Pakistan J Med Sci. 2016;32(3):550-4.
27. Yavuz T, Eraslan O. The effect of silane applied to glass ceramics on surface structure and bonding strength at different temperatures. J Adv Prosthodont. 2016 [cited 2015 Oct 12];8(2):75. Available from: http://synapse.koreamed.org/DOIx.php?id=10.4047/jap.2016.8.2.75
28. Tian T, Tsoi JKH, Matinlinna JP, Burrow MF. Evaluation of microtensile bond strength on ceramic-resin adhesion using two specimen testing substrates. Int J Adhes Adhes. 2014 [cited 2015 Oct 12];54:165-71. Available from: http://dx.doi.org/10.1016/j.ijadhadh.2014.06.003
29. Baratto SSP, Spina DRF, Gonzaga CC, da Cunha LF, Furuse AY, Filho FB, et al. Silanated surface treatment: Effects on the bond strength to lithium disilicate glass-ceramic. Braz Dent J. 2015;26(5):474-7.
30. Makishi P, André C, Silva JL, Bacelar-Sá R, Correr-Sobrinho L, Giannini M. Effect of Storage Time on Bond Strength Performance of Multimode Adhesives to Indirect Resin Composite and Lithium Disilicate Glass Ceramic. Oper Dent. 2016 [cited 2015 Oct 12];15-187-L. Available from: http://www.jopdentonline.org/doi/10.2341/15-187-L
31. Sebastião Garboza C, Berger SB, Guiraldo RD, Paula A, Fugolin P, Gonini-Júnior A, et al. Influence of Surface Treatments and Adhesive Systems on Lithium Disilicate Microshear Bond Strength. 2016;27:458-62.
32. Guarda GB, Correr AB, Goncalves LS, Costa AR, Borges GA, Sinhoreti MA, et al. Effects of surface treatments, thermocycling, and cyclic loading on the bond strength of a resin cement bonded to a lithium disilicate glass ceramic. Oper Dent. 2013 [cited 2015 Sep 2];38(2):208-17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22856682%5Cn
33. Lise D, Perdigão J, Van Ende A, Zidan O, Lopes G. Microshear Bond Strength of Resin Cements to Lithium Disilicate Substrates as a Function of Surface Preparation. Oper Dent. 2015 [cited 2015 Sep 2];150306070625005. Available from: http://www.jopdentonline.org/doi/10.2341/14-240-L
34. Wahsh MM, Ghallab OH. Influence of different surface treatments on microshear bond strength of repair resin composite to two CAD/CAM esthetic restorative materials. Tanta Dent J. 2015 [cited 2015 Sep 2];12(3):178-84. Available from: http://dx.doi.org/10.1016/j.tdj.2015.06.001
35. Yavuz T, Ozyilmaz OY, Dilber E, Tobi ES, Kilic HS. Effect of Different Surface Treatments on Porcelain-Resin Bond Strength. J Prosthodont. 2015 [cited 2015 Sep 22]:1-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26480029
Recibido: 18 de abril de 2017.
Aprobado: 24 de mayo de 2017.