Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Estomatología
versión On-line ISSN 1561-297X
Rev Cubana Estomatol vol.55 no.2 Ciudad de La Habana abr.-jun. 2018
PRESENTACIÓN DE CASO
Dermal filler-related oral lesion
Lesión bucal relacionada con relleno dérmico
Francisco Samuel Rodrigues Carvalho,I Eduardo Costa Studart Soares,II Victor Pinheiro Feitosa,III Mário Rogério Lima Mota,II Ana Paula Negreiros Nunes Alves,II Fábio Wildson Gurgel CostaII
I Fortaleza University (UNIFOR). Fortaleza, Brazil.
II Federal University of Ceará. Fortaleza, Brazil.
III Paulo Picanço School of Dentistry. Fortaleza, Brazil.
ABSTRACT
Introduction: Oral adverse reactions related to natural dermal fillers may originate from infiltration techniques, giving rise to swellings, nodule formation doe to local material entrapment, or displacement or migration of the material used.
Objective: describe a case of orofacial foreign body reaction in an elderly patient.
Case report: a 65-year-old woman was referred for oral evaluation complaining of an intraoral lesion present for 15 days. Intraoral examination revealed a mobile nodule in the lower lip left portion. After the initial consultation, incisional biopsy was performed under local anesthesia and the surgical specimen was sent for histopathological analysis. Intraoral examination revealed a soft mobile nodule in the lower lip left portion. Clinical pathological and Raman microspectroscopy analysis led to a final diagnosis of calcium hydroxyapatite dermal filler-related reaction.
Conclusions: this case reinforces the possibility of dermal filler-related mucosal tissue reactions in oral cavity soft tissues.
Keywords: dermal fillers; oral pathology; Raman spectroscopy.
RESUMEN
Introducción: las reacciones adversas bucales relacionadas con rellenos dérmicos naturales pueden originarse a partir de técnicas de infiltración, lo que ocasiona inflamaciones, formación de nódulos por atrapamiento de material local o desplazamiento o migración del material usado.
Objetivo: describir un caso de reacción a cuerpo extraño bucofacial en un adulto mayor.
Presentación del caso: una mujer de 65 años de edad fue remitida para evaluación bucal por presentar una lesión intrabucal durante 15 días. El examen intrabucal reveló un nódulo móvil en la porción izquierda del labio inferior. Después de la consulta inicial, se realizó una biopsia incisional bajo anestesia local y se envió la muestra quirúrgica para su análisis histopatológico. El examen intrabucal reveló un nódulo móvil suave en la porción izquierda del labio inferior. El análisis clínico-patológico y de la micro-espectroscopia de Raman condujeron a un diagnóstico final de reacción de hidroxiapatita cálcica relacionada con el relleno dérmico.
Conclusiones: este caso refuerza la posibilidad de reacciones cutáneas relacionadas con los rellenos dérmicos en los tejidos mucosos de la cavidad bucal.
Palabras clave: rellenos dérmicos; enfermedad bucal; espectroscopia Raman.
INTRODUCTION
Orofacial foreign body reactions following cosmetic procedures may present as an asymptomatic swelling or be related to pain, burning sensation, pruritus, or erythema.1-8 Dermal filler-related adverse reactions may originate from infiltration techniques, giving rise to swellings, nodule formation from local material entrapment, or displacement or migration of the material due to muscular activity.2 In this context, foreign body reactions in mucosal tissues should be considered even when using apparently safety materials.7
Diverse methods have been proposed in order to identify the specific cosmetic filler deposited into the affected tissue, including energy-dispersive x-ray analysis and high-frequency ultrasonography. 4 However, modern procedures using Raman micro-spectroscopy have been recently used in orofacial foreign body reactions due to the possibility of characterizing specific molecules based on its specific vibrational states.2
This study describes a rare case of orofacial foreign body reaction related to dermal filler containing calcium hydroxyapatite in an older patient and the clinical, histopathological, and spectroscopy aspects.
CASE REPORT
A 65-year-old woman was referred for oral evaluation at the Clinic of Stomatology, Federal University of Ceará (Brazil) complaining of painless intraoral lesion present for 15 days. The patient reported that the nodules arose spontaneously without symptoms of oral infection. Her previous medical history was unremarkable, and the patient did not recall any dental or medical procedures related to the lesion development.
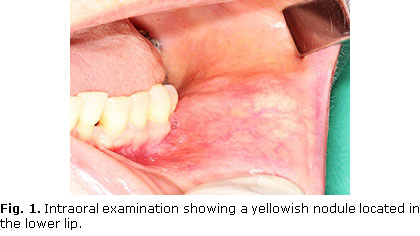
Intraoral examination revealed a mobile nodule in the lower lip left portion (Fig. 1). This lesion measured approximately 1 cm, and it was observed a firm consistency during intraoral evaluation. The nodule was recovered by a non-ulcerated yellowish mucosa. The adjacent perioral region was unremarkable (Fig. 1).
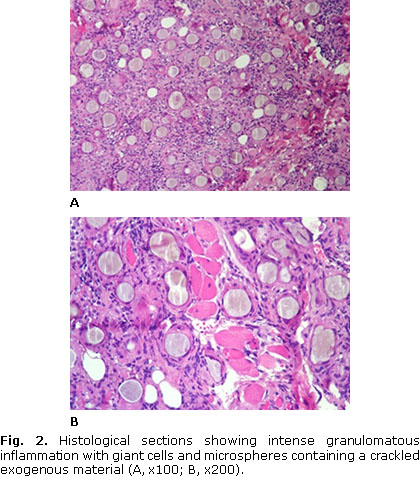
After the initial consultation, an incisional biopsy was held under local anesthesia and the surgical specimen was sent for histopathological analysis. Microscopically, it was observed intense granulomatous inflammation with giant cells associated with microspheric foreign bodies showing a crackled appearance (Fig. 2). In order to study the nature of these round and uniformly sized deposits, both von Kossa stain and Raman micro-spectroscopy were performed. These spherical foreign bodies showed a brown-gray color after von Kossa staining (Fig. 3). Raman micro-spectroscopy study was conducted with vibrations in the 10-4 000 cm-1 spectral region. The spectrometer was calibrated on silica. The exposure time for every spot was 3 seconds with 10 scans accumulation. Spectrophotometric showed a strong peak at 960 cm-1 and a phosphate isoform band at 422 cm-1, representing the P-O bonds of hydroxyapatite (Fig. 3). Thus, the final diagnosis was foreign body reaction to calcium hydroxyapatite. Although we were uncertain about the complete filler removal, it was requested to the patient a periodic visit for accomplishment.
DISCUSSION
Nowadays, a broad of surgical and non-surgical procedures has been described to improve aging changes, such as skin wrinkles, protuberant nasolabial folds, deep furrows, and lip thinning. The use of dermal filler has increased in patients seeking dental care, but the substances used for this procedure have been related to a significant risk of adverse reactions (e.g. pain, swelling, ecchymosis, erythema, dyschromia, skin texture changes, and orofacial nodules), whose manifestations can simulate intra-oral diseases.1 The most common cosmetic dermal fillers have been divided into two material type-related categories:2 1) natural (bovine collagen, human-derived collagen, hyaluronic acid, poly-L-lactic acid, and calcium hydroxyapatite); 2) synthetic (silicone, polyacrylamide, polymethy1 methacrylate, polyalkylimide, and polyvinylhydroxide).
Clinicians should know the possibility of oral reactions related to natural injectable dermal fillers. Radiesse® is a gel containing calcium hydroxyapatite (30 %) and carboxymethylcellulose (70 %). Although it is formulated with a biomaterial, nodules may occur within 6 to 12 weeks after lip injection.7 In older persons, a PubMed updated search for cases of Radiesse® oral reactions yielded only 3 publications to date.2,9,10 Lower lip was the most cited region. In a recent review of the literature published in 2017, Radiesse®-related adverse events were observed in only 3 % of the 5 081 treatments performed with this soft tissue filler.11
In the present case, the histopathologic diagnosis was obtained based on the morphological appearance, which has been documented in the literature. However, Artecoll® (polymethy1 methacrylate) and Evolution® (polyvinylhydroxide) share some microscopic similarities with Radiesse.7 Thus, von Kossa staining and spectrometric evaluation were important to clarify the foreign body nature. For the second time in the literature, Raman micro-spectroscopy was used aiming to characterize the foreign body microspheres. The present analysis was similar to Owosho et al.2 We observed a strong pick at 960 cm-1, signalizing a P-O bond compatible with calcium hydroxyapatite.
Management of individuals with Radiesse® oral adverse reactions should consider the number of lesions (single or multiple; isolated or bilateral), presence or absence of clinical symptoms, location, and size, as well as the remaining pathologic tissue after an incisional biopsy. Presently, the first surgical appointment resulted in an esthetic area according to the patient perception after the complete healing process. Postoperative patient visit was uneventful. However, complete remission of the lesions is not always achieved.1
In conclusion, the oral health is an important field of knowledge for dental and non-dental professionals. Although Radiesse® has been considered a safe dermal filler, the present report reinforces the possibility of mucosal tissue reactions following its application.
Conflict of interest
No conflict of interest exists among the participants in the study.
BIBLIOGRAPHIC REFERENCES
1. Costa FW, Teixeira LH, Carvalho FS, Chaves FN, Turatti E, Ribeiro TR, et al. Bilateral oral nodules after the use of a dermal filler containing polymethylmethacrylate microspheres in an older woman. J Am Geriatr Soc. 2014;62(3):587-8.
2. Owosho AA, Bilodeau EA, Vu J, Summersgill KF. Orofacial dermal fillers: foreign body reactions, histopathologic features, and spectrometric studies. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):617-25.
3. Ferneini EM, Beauvais D, Aronin SI. An Overview of Infections Associated With Soft Tissue Facial Fillers: Identification, Prevention, and Treatment. J Oral Maxillofac Surg. 2017;75(1):160-6.
4. Feio PS, Gouvêa AF, Jorge J, Lopes MA. Oral adverse reactions after injection of cosmetic fillers: report of three cases. Int J Oral Maxillofac Surg. 2013;42(4):432-5.
5. Requena C, Requena L, Alegre V, Serra C, Llombart B, Nagore E, et al. Adverse reaction to silicone simulating orofacial granulomatosis. J Eur Acad Dermatol Venereol. 2015;29(5):998-1001.
6. Kehily E, Hayes M, McCreary C. Adverse reactions to facial dermal fillers: a case report. J Ir Dent Assoc. 2015;61(1):36-9.
7. Moulonguet I, Arnaud E, Bui P, Plantier F. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35(3):e37-40.
8. Shahrabi-Farahani S, Lerman MA, Noonan V, Kabani S, Woo SB. Granulomatous foreign body reaction to dermal cosmetic fillers with intraoral migration. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):105-10.
9. Abstracts presented at the Annual Meeting of the American Academy of Oral and Maxillofacial Pathology, April 29-May 3, 2011. Oral Surg Oral Med Oral Pathol Oral Radiol. 2011;112:e45.
10. Daley T, Damm DD, Haden JA, Kolodychak MT. Oral lesions associated with injected hydroxyapatite cosmetic filler. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(3):107-11.
11. Kadouch JA. Calcium hydroxylapatite: A review on safety and complications. J Cosmet Dermatol. Mar 1, 2017. doi: 10.1111/jocd.12326.
Recibido: 16 de junio de 2016.
Aprobado: 3 de marzo de 2018.