Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión On-line ISSN 1561-2988
Rev Cubana Farm v.45 n.1 Ciudad de la Habana ene.-mar. 2011
ARTÍCULOS ORIGINALES
Efficacy and safety of ior® LeukoCIM (G-CSF) in patients with neutropenia after chemotherapy
Eficacia y seguridad del ior® LeukoCIM (FEC-G) en pacientes con neutropenia posquimioterapia
Leslie Pérez RuizI; Ana María Ramos CedeñoII; Julio Dámaso Fernández ÁguilaIII; Tamara Guerra AlfaroIV; Maritza Cabrera ZamoraIV; Luciano Julián Pascau IllasV
ILicenciada en Farmacia. Asistente Investigadora Agregada. Center of Molecular Immunology (CIM). Havana City, Cuba.
IIEspecialista de II Grado en Farmacología. Máster en Ciencias. Profesora Auxiliar. Clinical Trials Department Medicine School. Cienfuegos, Cuba.
IIIEspecialista de II Grado en Hematología. Asistente. Hospital "Gustavo Aldereguía Lima". Cienfuegos, Cuba.
IVEspecialista de II Grado en Hematología. Hospital "Gustavo Aldereguía Lima". Cienfuegos, Cuba.
VLicenciado en Farmacia. Máster en Ciencias. Center of Molecular Immunology (CIM). Havana City, Cuba.
ABSTRACT
Neutropenia and infections are the most restrictive side effects during chemotherapy application. The granulocytic colonies stimulating factor activates the neutrophils, shortens the neutropenic period and can be effective against the potential risk of infection. The purpose of this study was to evaluate the efficacy and safety of LeukoCIM® (CIMAB, Havana). A retrospective observational study was carried out with data from the patients with neutropenic episodes enrolled in the open-label, non-randomized, multicenter, phase IV clinical trial. These patients were from Gustavo Aldereguía Lima hospital. They had been evaluated for one year. Demographic information, clinical data and side effects were analyzed. As prophylaxis indication LeukoCIM® was administrated 24-72 h after the last chemotherapy dose and as treatment when neutropenia was diagnosed. In both cases, a daily single 300 µg dose was administrated subcutaneously. The application of the next chemotherapy cycle on time was the main variable of response and the product safety was assessed by measuring the side effects. Forty seven patients with 95 neutropenic episodes were enrolled. The 82.1 % of episodes received their next chemotherapy cycle on time. The most frequent side effects were: bone pain and fever (11.2 % respectively), hyperuricemia (9.2 %), leukocytosis and neutrophilia (7.1 %) and increased LDH (6.1 %). LeukoCIM® was effective in patients receiving chemotherapy, because it accelerated neutrophil recovery, decreased the incidence of febrile neutropenia and improved delivery of protocol doses of chemotherapy on time. Additionally, this product was considered safe for the studied patients since just known adverse events were reported.
Key words: Neutropenia, neutrophils, hematologic diseases, side effects, clinical trials.
RESUMEN
La neutropenia y las infecciones constituyen los eventos adversos más limitantes en la aplicación de quimioterapia. Los factores estimulantes de colonias de granulocitos activan los neutrófilos, acortan el periodo neutropénico y pueden ser efectivos contra los riesgos potenciales de infección. El propósito de este estudio fue evaluar la efectividad y seguridad del LeukoCIM® (CIMAB, La Habana). Se realizó un estudio retrospectivo, observacional con los datos de los pacientes incluidos en el ensayo clínico fase IV abierto, no aleatorizado y multicéntrico. Estos pacientes provenían del Hospital Gustavo Aldereguía Lima y se evaluaron durante un año. Se analizaron los datos demográficos, clínicos y de seguridad. Como profilaxis el fármaco fue administrado de 24-72 h después de la última dosis de quimioterapia y como tratamiento cuando la neutropenia había sido diagnosticada. En ambos casos la dosis única diaria fue de 300 µg por vía subcutánea. La administración del próximo ciclo de quimioterapia en tiempo resultó la variable principal de respuesta y la seguridad del producto se evaluó midiendo los eventos adversos. Se incluyeron 47 pacientes con 95 episodios neutropénicos. El 82,1 % de episodios recibió su próximo ciclo de quimioterapia en tiempo. Los eventos adversos más frecuentes fueron: dolor óseo y fiebre (11,22 % respectivamente), hiperuricemia (9,2 %), leucocitosis y neutrofilia (7,1 %) e incremento de LDH (6, 1%). LeukoCIM® resultó efectivo, pues aceleró la recuperación del número de neutrófilos, disminuyó la incidencia de neutropenia febril y permitió administrar las dosis de quimioterapia en tiempo según el protocolo. También se consideró seguro en la serie estudiada, pues solo reportó eventos adversos conocidos.
Palabras clave: Neutropenia, neutrófilos, enfermedades hematológicas, eventos adversos, ensayos clínicos.
INTRODUCTION
At the present, the treatment of cancer is based in the antineoplastic chemotherapy. One of the main side effects of chemotherapy drugs is a reduction in the number of neutrophils. This makes the body less able to fight against infection. There is a risk that the patient could develop a serious infection, which might have to be treated in hospital.1-8 Myelosuppression, particularly neutropenia, represents the major dose-limiting toxicity of systemic cancer chemotherapy. Importantly, studies in early stage of breast cancer and Non-Hodgkin Lymphoma (NHL) showed that severe and febrile neutropenia are the major causes of chemotherapy dose reductions and delays that reduce relative dose intensity and thus reduce the potential for prolonged disease-free and overall survival.9 Researchers from the University of Rochester, the University of Washington and Duke University have concluded that the prophylactic use of granulocyte-colony stimulating factor (G-CSF) reduces febrile neutropenia and early deaths due to infections in adult patients receiving chemotherapy.10
Endogenous granulocyte colony-stimulating factor is a lineage-restricted colony-stimulating factor that principally affects the proliferation, differentiation, and activation of committed progenitor cells of the neutrophil-granulocyte linage. In addition, endogenous G-CSF enhances certain functions of mature neutrophils, including phagocytosis, chemotaxis, and antibody-dependent cellular cytotoxicity. It is an 18 000 Dalton glycoprotein produced by monocytes, fibroblasts, and endothelial cells. G-CSF has been shown to have minimal direct in vivo or in vitro effects on the production of other hematopoietic cell types.11-17 G-CSF is also known as colony-stimulating factor 3 (CSF 3).
The administration of prophylactic G-CSF formulations to patients receiving chemotherapy accelerates neutrophil recovery, decreases the incidence of febrile neutropenia and improves delivery of protocol doses of chemotherapy.10,18-26 Chemotherapy can cause myelosuppression and unacceptably low levels of white blood cells, making patients prone to infections and sepsis.1-2,27
Researchers from Poland and Germany reported that adding Neupogen® (Filgrastim) or Granocyte® (Lenograstim) reduced the incidence of neutropenic fever and other symptoms associated with Taxotere® (docetaxel), Adriamycin® (doxorubicin) and Cytoxan® (cyclophosphamide) (TAC). The addition of Neupogen® or Granocyte® reduced the percentage of patients with clinically relevant symptoms from 64 to 46 %.28
The American Society of Clinical Oncology (ASCO) reports yearly the results of different investigations about the use hematopoietic colony-stimulating factors. ASCO convened an Update Committee composed of the original Expert Panel and select ad hoc members to present the 2006 evidence-based clinical practice guideline update for the use of these kinds of drugs. The Expert Panel listed three reasons to value the use of the G-CSF in neutropenic patients.29
1. Therapeutic intervention with CSF can help reduce the incidence of infectious episodes and infection-related morbidity and mortality.
2. CSF could be used to shorten the neutropenic period.
3. It is recommended in patients with mielotoxicity, pneumonitis and mucositis, due to chemotherapy or radiotherapy.
The safety of the different colony stimulating factor was studied in many investigations. The more common side effects are: bone pain, injection site pain, headache, arthralgia, myalgia, back pain,6,30 fever, chills, body aches, flu symptoms, nausea, vomiting, loss of appetite, diarrhea, constipation, hair loss, itchy skin.5-7,30-36
One or more of the following signs and symptoms were observed shortly after the subcutaneous injection of G-CSF in patients with chronic myeloid leukemia undergoing allogeneic peripheral blood stem cell transplantation: dyspnea, chest pain, nausea, hypoxemia, diaphoresis, anaphylaxis, syncope and flushing.33
A new recombinant G-CSF preparation was recently obtained by recombinant DNA technology. It is a highly purified methionyl form of E. coli expressed recombinant human G-CSF containing 175 amino acid residues that was developed by the Center of Molecular Immunology (CIM, Havana, Cuba, and is currently marketed by CIMAB.SA.37,38
LeukoCIM® regulates the production and release of functional neutrophils from the bone marrow and controls their proliferation, differentiation and other cellular functions. LeukoCIM® results in a dose dependent increase in neutrophil counts which return to baseline within 4 days of discontinuation of it. This product is used in the treatment of patients with neutropenia due to chemotherapy.38
According to Ducongé et al., LeukoCIM®, is pharmacokinetic comparability with Neupogen®, Hoffman-La Roche, licensed by Amgen.37
This paper is about the efficacy and safety of ior® LeukoCIM in primary or secondary prophylaxis or treatment in neutropenic patients.
METHOD
A phase IV, open-label, non-randomized, multicenter clinical trial was carried out in neutropenic patients due to chemo/radiation antineoplastic therapy.
Sample size
The number of subjects to the clinical trial was calculated stratified as follows:
Primary prophylaxis: Hypothesis: To increase the value of ANC³ 1.5 x 109/ L in the 75 % of the patients. Assuming error type I (a= 0,05) and error type II (b= 0,2) with 85 % of reference, in order to answer the hypothesis, it needs 91 patients and taking to account 10 % of losing the final number was 101 patients.
Secondary prophylaxis: Hypothesis: To increase the value of ANC³ 1.5 x 109/L in the 65 % of the patients. Assuming error type I (a= 0,05) and error type II (b= 0,2) with 75 % of reference, in order to answer the hypothesis, it needs 125 patients and taking to account 10 % of losing the final number was 138 patients.
Treatment: Hypothesis: To increase the value of ANC³ 1.5 x 109/L in the 80 % of the patients. Assuming error type I (a= 0.05) and error type II (b= 0.2) with 70 % of reference, in order to answer the hypothesis, it needs 119 patients and taking to account 10 % of losing the final number was 133 patients.
The total number of sample size for the clinical trial was 372 patients, but we used in this work the subset of patients included in the "Dr. Gustavo Aldereguía Lima" University Hospital.
From it, 95 neutropenic episodes were studied picked up in clinical records and Case Report Forms (CRF) belonging to the all the patients (47) treated in the "Dr. Gustavo Aldereguía Lima" University Hospital, Cienfuegos during one year. With this data a retrospective observational descriptive study was carried out. The following variables were analyzed: age, gender, ethnicity, indication for G-CSF administration, values of absolute neutrophil count (ANC), and the value of uric acid, the number of doses administered, the regimen of treatment and the interruption of treatment.
The protocol was approved according to International Conference Harmonization (ICH) by the Institutional Review Board (IRB) of the "Dr. Gustavo Aldereguía Lima" University Hospital, Havana, and by the Cuban Regulatory Authority. The informed consent to participate, were included and all patients singed it.
Also, the side effects were analyzed. They were classified according to WHO Toxicity Criteria18,39 and their relation of causality was classified according to Karch Lasagna algorithm.40
G-CSF formulation
LeukoCIM®, has been produced following the standard of quality for injectable formulations, TRS 823 and 822 GMP regulations for pharmaceutical and biological drug products, respectively; and also following the Cuban norm 16/2000 from CECMED, Havana, Cuba (i.e. The Cuban Regulatory Agency identified as The State Center for Drug Quality Control).37 Each vial contains 300 (G30) mg/mL (specific activity: 108 UI/mg proteins) of sterile recombinant human G-CSF, 50 mg sorbitol, 0.04 mg polysorbate 80, 0.59 mg sodium acetate and water for injection to complete 1 mL.38
LeukoCIM® was administrated in primary/secondary prophylaxis by subcutaneous way in the deltoid region 24-72 h after the last chemotherapy dose during 7-10 days according investigator´s criteria. The daily single dose was 300 µg.38
Its use as treatment in patients with febrile neutropenia (ANC£ 1 x 109/L) or non febrile neutropenic (ANC£ 0.5 x 109/L) until ANC at least 2-3 x109/L. The daily single dose was 300 µg by subcutaneous way in deltoid region.38
The administration of the next chemotherapy or radiation therapy cycle on time was the main variable of response. The value of ANC and time to absolute neutrophil count recovery were also evaluated.
The results were introduced in a data base and they were analyzed by SPSS version 13.0 for windows used descriptive statistical and they were express in number and percent. Mean, minimum and maximum values were determinate. Also Pearson l2 Test was determinate to the main variable of response.
RESULTS
Forty seven patients with 95 neutropenic episodes were enrolled. Twenty eight patients (59.6 %) had one neutropenic episode, seven patients (14.9 %) had three episodes, five patients (10.6 %) had two episodes, four patients (8.5 %) had four episodes, with five, seven and eight episodes had one patient (2.1 %) respectively. In the 8.4 % (eight patients) of the episodes the indication was noted as primary prophylaxis, in 31.6 % of episodes (thirty patients) as secondary and in 60% of episodes (fifty seven patients) as treatment.
The median age of the patients was 52 years. There were 29 females (62 %) and 18 males (38 %). White skin was the predominant color (77.9 %), followed by brown (12.6 %) and black (9.5 %). The more common patient's diagnoses by neutropenic episodes were: acute non-lymphoid leukemia (34.7 %), non-Hodgkin's lymphoma (15.8 %) and Hodgkin's lymphoma (13.7 %) (table 1).
Table 1. Distribution of episodes by patients diagnoses and indication
| Patients diagnoses | Prophylaxis No. episodes | Treatment No. episodes | Total episodes |
| Acute non-lymphoid leukemia | 9 | 24 | 33 |
| Acute lymphoid leukemia | 0 | 3 | 3 |
| Leukemia biphenotypic acute | 0 | 2 | 2 |
| Non-Hodgkins lymphoma | 5 | 10 | 15 |
| Hodgkins lymphoma | 7 | 6 | 13 |
| Multiple myeloma | 1 | 0 | 1 |
| Myelodysplastic syndrome | 7 | 1 | 8 |
| Breast carcinoma | 6 | 4 | 10 |
| Ovarian adenocarcinoma | 3 | 4 | 7 |
| Laryngeal carcinoma | 0 | 1 | 1 |
| Epidermoide lung carcinoma | 0 | 1 | 1 |
| Rectus carcinoma | 0 | 1 | 1 |
| Total | 38 | 57 | 95 |
Source: Clinical records and CRF from: An open-label, no-randomized, multicenter, phase IV clinical trial Efficacy and safety of LeukoCIM® in neutropenia post chemotherapy.
Gustavo Aldereguía University Hospital, Cienfuegos, Cuba. 2007.
The initial mean uric acid level was 266.34 and the final was 309.87.
In 9 episodes there was interruption of treatment (9.5 %). Fifty and half percent received the treatment in ambulatory way (48 episodes), whereas 48.4 % was hospitalized (47 episodes).
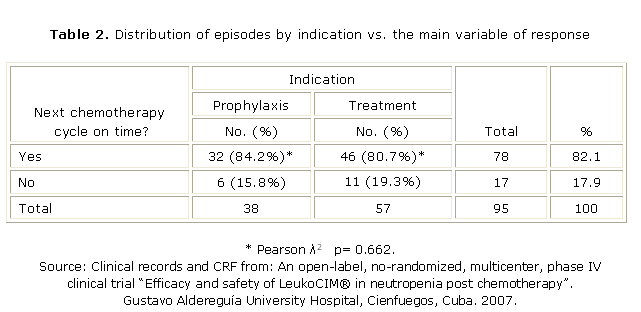
From 38 patients that were enrolled in the prophylaxis stratus, 32 received their next chemotherapy cycle on time (84.2 %). In the other hand, from 57 episodes included in the treatment stratus, 46 received their next chemotherapy cycle on time (80.7 %) (table 2). According to Pearson l2 Test, there was no association between the main variable of response and the stratus p= 0.662.
About the seventeen patients that no received their next chemotherapy cycle on time, 2.1 % was due to prolonged neutropenia, thrombocytopenia and pneumonia respectively, 8.4 % because of death and 3.1 % due to withdrawal (table 3).
The initial mean value of ANC was 1.49 cells/L and the final was 5.51 cells/L.
The mean dose number administered to obtain the recovery of absolute neutrophil count was 6.69, so the recovery of ANC was approximately in a week; therefore the majority of the patients received their next chemotherapy cycle on time (82.1 %) (table 2). The final value of the ANC allowed measuring the main variable of response.
About the safety of the LeukoCIM®, from 95 neutropenic episodes, 55 involved side effects (58 %) there were distributed in 28 patients (59.6 %). These adverse events were distributed as follows: with 1 event 32.6 % of episodes; 2 events 18.9 % of episodes; 3 events 2.1 % of episodes; 4 events 1.0 % of episodes; 6 events 2.1 % of episodes; and 8 events 1.0% of episodes (table 4).
Relating to toxicity of the product, 74 episodes (75.5 %) had mild intensity, 13 (13.3 %) were moderate, 8 (8.1 %) were classified as grave and 3 (3.1 %) were severe. Concerning to the relation of causality between LeukoCIM® and side effects, 60 episodes (61.2 %) were classified as possible, 24 (24.5 %) were probable, 11 (11.2 %) were very probable and 3 (3.1 %) were classified as remote.
Eight patients died, this was classified grave and possible due to their illness and not because of the used of LeukoCIM®.
LeukoCIM® was generally well tolerated, and only rarely were the adverse effects severe enough to require discontinuation of the treatment.
The side effects reported most frequently were: bone pain and fever 11 episodes (11.2 % respectively), hyperuricemia 9 episodes (9.2 %), leukocytosis and neutrophilia 7 episodes (7.1 %), increased of Lactate dehydrogenase (LDH) 6 episodes (6.1 %), thrombocytopenia 5 episodes (5.1 %), headache and nauseas 4 episodes (4.1 %) respectively, hypotension 3 episodes (3.1%), injection site pain, asthenia, myalgia and anorexia 2 episodes (2.0 %) each one. The other side effects such as: vomiting, retinal hemorrhage, upper digestive bleeding, itchy skin, melena, epistaxis, pruritus, chills, renal insufficiency, weight loss, eritema, drowsiness, transpiration skin, cellulitis and increased of leukocyte alkaline phosphatase (LAP) 1 episode (1.0 %) each one (table 5).
| Side effects | Prophylaxis | Treatment | Total | % |
| Bone pain | 5 | 6 | 11 | 11.22 |
| Fever | 8 | 3 | 11 | 11.22 |
| Hyperuricemia | 4 | 5 | 9 | 9.18 |
| Death | 0 | 8 | 8 | 8.16 |
| Leukocytosis | 4 | 3 | 7 | 7.14 |
| Neutrophilia | 4 | 3 | 7 | 7.14 |
| Increased of LDH | 4 | 2 | 6 | 6.12 |
| Trombocytopenia | 4 | 1 | 5 | 5.10 |
| Nauseas | 0 | 4 | 4 | 4.08 |
| Headache | 0 | 4 | 4 | 4.08 |
| Hypotension | 3 | 0 | 3 | 3.06 |
| Injection site pain | 0 | 2 | 2 | 2.04 |
| Asthenia | 2 | 0 | 2 | 2.04 |
| Myalgia | 2 | 0 | 2 | 2.04 |
| Anorexia | 0 | 2 | 2 | 2.04 |
| Vomiting | 0 | 1 | 1 | 1.02 |
| Retinal hemorrhage | 0 | 1 | 1 | 1.02 |
| Upper digestive bleeding | 0 | 1 | 1 | 1.02 |
| Itchy skin | 1 | 0 | 1 | 1.02 |
| Melena | 0 | 1 | 1 | 1.02 |
| Epistaxis | 0 | 1 | 1 | 1.02 |
| Pruritis | 1 | 0 | 1 | 1.02 |
| Chills | 1 | 0 | 1 | 1.02 |
| Renal insufficiency | 0 | 1 | 1 | 1.02 |
| Weight loss | 1 | 0 | 1 | 1.02 |
| Erythema | 1 | 0 | 1 | 1.02 |
| Drowsiness | 1 | 0 | 1 | 1.02 |
| Transpiration skin | 1 | 0 | 1 | 1.02 |
| Cellulitis | 0 | 1 | 1 | 1.02 |
| Increased of LAP | 1 | 0 | 1 | 1.02 |
| Total | 48 | 50 | 98 | 100 |
Source: Clinical records and CRF from: An open-label, no-randomized, multicenter, phase IV clinical trial Efficacy and safety of LeukoCIM® in neutropenia post chemotherapy.
Gustavo Aldereguía University Hospital, Cienfuegos, Cuba. 2007.
Thirteen episodes (13.6 %) received concomitant treatment. The more common drugs were: acetaminophen and dipirona in order to treat fever, chills, headache and bone pain, gentamicin to treat sepsis and cytotoxic anticancer agents such as: andriamycin, mitoxantrone and cytosine arabinosid.
DISCUSSION
The use of LeukoCIM® has permitted the administration of an effective dose of chemotherapy and the majority of the patients received the next cycle on time, so the product was effective as other G-CSFs.9,10,28 Its pharmacokinetic is comparability with Neupogen®,37 also the study with a glycosylated CHO-derived G-CSF has reported that the bioavailabilities of different G-CSF molecular forms are similar.41
Data on the safety of the treatment with G-CSF formulations have been reported with standard doses of chemotherapy or high doses with or without hematopoietic progenitor's transplant.32,34,36,42-46
In our study, the levels of the uric acid were monitored before and after the use of the LeukoCIM®, hyperuricemia was detected in the 9.18 % of episodes. These spontaneously reversible elevations in uric acid were generally mild to moderate. At the end of the treatment the patients recovered their normal uric acid value in a week, this agrees with the clinical trial carried out with Wilford et al.38 and with the literature consulted.34,47
The bone pain was manifested frequently. LeukoCIM®-induced bone pain usually can be effectively prevented or treated with non-opioid oral analgesics (e.g. acetaminophen).5-7,46 In severe cases, opioid analgesics may be use.47 This result agrees with clinical studies reviewed, which have shown good profile of security and tolerance for LeukoCIM®38 and others G-CSFs;10,45,46 according to these reviews bone pain mild-moderate 10 %, bone pain severe 5 % and local reactions on injection site 2-5 % in patients treated with subcutaneousinjection.24,48 Other side effects like: fever, neutrophilia, leukocytosis, increased of LDH, erythema, chills, itchy skin, hypotension and thrombocytopenia are also reported in literature.5-7 It could be prescribe painkillers such as acetaminophen to help reduce temperature and prevent chills.5-7,31
Besides that, another side effects as difficulty breathing, sudden or severe pain in the left upper stomach spreading up to the shoulder, constipation, diarrhea, hair loss, white patches or scores inside the mouth or on the lips and dysuria have been reported,5-7,31 also chest pain, hypoxemia, diaphoresis, anaphylaxis, syncope and flushing33 but not in this study.
In our study, transient decreases in blood pressure (< 90/60 mmHg), which did not require clinical treatment, was reported in 3 cases.
The increased in neutrophil counts during mobilization, consistent with the biological effects of LeukoCIM® and no sequelae were associated with any grade of leukocytosis.
About renal insufficiency, the relationship of this event to LeukoCIM® remains unclear since it occurred in patient with culture-proven infection with clinical sepsis who was receiving potentially nephrotoxic antibacterial therapy (Gentamicin).
One patient, with acute non-lymphoid leukemia, had retinal hemorrhage and upper digestive bleeding during thrombocytopenia due to myeloablative therapy. In relation to the eight dead patients, they died because their illness and not because of LeukoCIM® as we can confirm according to the necropsy results. Four patients died because Bacterial bronchopneumonia (direct cause) and Non-Hodgkin's lymphoma (basic cause). Three patients died because bilateral bronchopneumonia (direct cause) and acute non-lymphoid leukemia (basic cause). One patient died because acute respiratory insufficiency (direct cause) and multiple myeloma (basic cause).
These results agree with the literature consulted, up to the moment any death has been related to G-CSF used.5-7,31,33
No evidence of interaction by LeukoCIM® with other drugs was observed in the course of clinical trial. The patients received in addition to LeukoCIM®: acetaminophen, dipirona, gentamicin and cytotoxic anticancer agents (andriamycin, mitoxantrone and cytosine arabinosid). According to the literature consulted the administration of Molgramostim (Growgen®) with cytotoxic and antiretroviral agents could produced thrombocytopenia49,50 and Filgrastim with lithium could produce leukocytosis.51,52
CONCLUSIONS
These data document the benefits of G-CSF in adult cancer patients receiving chemotherapy.
LeukoCIM® was effective to patients receiving chemotherapy, because accelerates neutrophil recovery, decreases the incidence of febrile neutropenia and improves delivery of protocol doses of chemotherapy on time. Also its use was safe in the studied patients, because it reported known adverse effects.
Acknowledgments
The study was financed by CIMAB Havana, Cuba (products, reagents), and the Ministry of Public Health of Cuba (hospital facilities and general medical care of the volunteers as in-patients). The authors wish to thank to the persons that gave them, their very helpful comments, which remarkably improved the quality of this paper and especially the 47 patients who served as volunteers for this study.
REFERENCES
1. Stull D, Bilmes R, Kim H, Fichtl R. Comparison of sargramostim and filgrastim in the treatment of chemotherapy-induced neutropenia. Am J Health-Syst Pharm. 2005;62:83-7.
2. Trillet-Lenoir V, Green J, Manegold C, Von Pawel J, Gatzemier U, Lebeau B, et al. Recombinant granulocyte colony stimulating factor reduce the infectious complication of cytotoxic chemotherapy. Eur J Cancer. 2003;29:319-24.
3. Sikora K, Advani S, Koroltchouk V, Magrath I, Levy L, Pinedo H, et al. Essential drugs for cancer therapy. A World Health Organization Consultation. Ann Oncol. 1999;10(4):385-90.
4. Pizzo PA. Management of fever in patients with treatment-induced neutropenia. N Engl J Med. 1993;328:1323-32.
5. Sweetman SC, Blake PS, McGlashan JM. Martindale. The Complete Drug Reference. 35th ed. London: Pharmaceutical Press; 2007.
6. British Medical Association and Royal Pharmaceutical Society of Great Britain. Drugs used in neutropenia. In: British National Formulary. 57th ed. British Medical Journal Publishing Group and Pharmaceutical Press. March, 2009. Accessed 01 April 2009. Available from: http://www.bnf.org/bnf/bnf/current/4926.htm
7. G-CSF (Neupogen®, Granocyte®, Neulasta®). Accessed 20 April 2009. Accessed 20 April 2009. Available from: http://www.cancerbackup.org.uk/treatments/supportivetherapies/haematopoieticgrowthfactors
8. William D, Dana W. Assessing the Relevant of Outcomes Data for Colony-Stimulating Factors. J Manager Care Pharm. 2000;6(2):144-50.
9. Timmer-Bone JN, de Boo TM, Smit HJ. Prevention of chemotherapy-induced febrile neutropenia by prophylactic antibiotics plus or minus granulocyte colonly-stimulating factor in small-cell lung cancer: A Dutch Randomized Study. J Clin Oncol. 2005;23:7974-84.
10. Kuderer NM, Dale DC, Crawford J. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol. 2007;25:3158-67.
11. American Society of Hospital Pharmacy. Filgrastim. American Hospital Formulary Service Drug Information. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1454-63.
12. Compendium of Pharmaceuticals and Specialities (CPS). Neupogen. Ottawa: Canadian Pharmaceutical Association; 2006.
13. Ulich TR, Castillo J, Souza L. Kinetics and mechanism of recombinant human granulocyte colony stimulating factor induced neutrophilia. Am J Pathol. 2001; 133:630-8.
14. Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005 Jan;105(2):830-7.
15. Sheridan W, Begley G, Juttner C. Effect of different doses and schedules of r-metHuG-CSF (filgrastim) dose on mononuclea r cell and PBPC collections and haematopoietic recovery after high dose chemotherapy (HDC) and infusion of r-metHuG-CSF mobilized peripheral blood progenitor cells (PBPC) without bone marrow. Blood. 1992;80:331a.
16. Sheridan W, Begley G, Juttner C. The impact of r-metHuG-CSF (filgrastim) dose on the mobilization of mononuclear and progenitor cells in peripheral blood in patients with malignancy. Blood. 1992;80:420a.
17. Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992;327:28-35.
18. Protocolo LNH-T. C-CHOP como tratamiento de 1ª línea en los Linfomas No Hodgkin-T periféricos, 2006:15. Citado 8 Abr 2009. Disponible en: http://www.lacohg.com/uploads/TBL_DOCTOSFF_44_1_31.CE.ISS.009_protocolSpanish.pdf
19. Cheng AC, Stephens DP, Currie BJ. Factor estimulante de colonias de granulocitos (G-CSF) como adyuvante de los antibióticos en el tratamiento de la neumonía en adultos. 2008. Disponible en: http://www.update-software.com
20. Koumakis G, Vassilomanolakis M, Barbounis V, Hatzichristou E, Demiri S, Plataniotis G, et al. Optimal Timing (Preemptive versus Supportive) of Granulocyte Colony- Stimulating Factor Administration following High-Dose Cyclophosphamide. Oncology. 1999;56(1):28-35.
21. Crawford J, Ozer H, Stoller R. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer (r-metHuG-CSF). N Engl J Med. 1991;325:164-70.
22. Stahel RA, Muller E, Pichert G. Dose intensification with autologous marrow support in high-risk lymphoma: acceleration of hematopoietic recovery and reduction of days of hospitalization with granulocyte colony-stimulating factor (G-CSF) in a randomized open-label trial (meeting abstract). Proc Am Soc Clin Oncol. 1992;11:331.
23. Ogawa M, Masaoka T, Takaku F. Effect of recombinant human granulocyte colony-stimulating factor (rhG-CSF) on leukopenia induced by chemotherapy for malignant lymphomas. Proc Am Soc Clin Oncol. 1990;9:76.
24. Hartmann L. Granulocyte Colony-Stimulating Factor in Severe Chemotherapy-Induced Afebrile Neutropenia. New Engl J Med. 1997 Jun;336(25):1776-80.
25. Tsukadaira A, Okubo Y, Takashi S, Kobayashi H, Kubo K. Repeated arthralgia associated with granulocyte colony stimulating factor administration Ann Rheum Dis. 2002;61:849-50.
26. Hollingshead L., Goa K. Recombinant Granulocyte Colony-Stimulating Factor (rG-CSF). A review of its pharmacological properties and prospective roles in neutropenic conditions. Drugs. 1991;42(2):300-30.
27. Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I. Marrow transplantation. Br J Haematol. 2002;82:589.
28. Martin M, Lluch A, Segui A. Toxicity and health-related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte-colony stimulating factor to the TAC regimen. An Oncol. 2006;17:1205-12.
29. Update of ASCO Practice Guideline Recommendations for the Use of White Blood Cell Growth Factors: Guideline Summary. J Clin Oncol. 2006; 2(4):3187-205.
30. Neulastim. Accessed 2008 Nov 25. Available from: http://www.medicamentos.com.mx/DocHTM/30007.htm
31. Drug information on line. Browse all medications. Filgastrim. Accessed 2009 Apr 8. Available from: http://www.drugs.com/MTM/filgrastim.html
32. Stern AC, Jones TC. The Side-Effect Profile of GM-CSF. Infection. 1992;20(2):S124.
33. Khoury H, Adkins D, Brown R, Vij R, Westervelt P, Trinkaus K, et al. Adverse side-effects associated with G-CSF in patients with chronic myeloid leukemia undergoing allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplantation. 2000;25:1197-201.
34. Decoster G, Rich W. Tolerability profile of r-metHuG-CSF (G-CSF NEUPOGEN). Eur J Cancer. 1991;27:233.
35. Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): The First 10 Years. Blood. 1996 Sep;88(6):1907-29.
36. Holmes F, Jones S, Shaughnessy J, Vukelja S, George T. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. An Oncol. 2002;13:903-9.
37. Duconge' J, Rodríguez L, Valenzuela C, Álvarez D, Ramírez O, de la Luz-Hernández KR, et al. Pharmacokinetic comparison of two recombinant human granulocyte colony-stimulating factor after subcutaneous administration in rabbits. Eur J Pharm Biopharm. 2005 Oct ;61(3):142-8.
38. Wilford M, Fernández JA, Mesa R, Muñio J, Figueredo I, Luna C, et al. Evaluación clínica y seguridad del ior G-CSF en pacientes con neutropenia grado III-IV por quimio y/o radioterapia. Estudio de extensión. En: V Congreso Nacional. VII Jornada Latinoamericana de Hematología, Inmunología y Medicina Transfusional. La Habana: Sociedad Cubana de Hematología, Inmunología y Medicina Transfusional, 2005. p. 16-20.
39. Miller AB. WHO Recommendations Toxicity Criteria. Cancer. 1981;47:210-1.
40. Laporte JR, Tognoni G. Principios de epidemiología del medicamento, 2ª ed. Barcelona: Salvat; 1993. p. 49-66.
41. Kuwabara T, Kobayashi S, Sugiyama Y. Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab Rev. 1996;28:625-58.
42. Stadtmauer EA, O'Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, et al. Conventional dose chemotherapy compared with high dose chemotherapy plus antilogous haematopoietic stem cell transplantation for metastatic breast cancer. N Engl J Med. 2001;325:1371-2.
43. Katzung BG. Farmacología básica y clínica. En: Chu E, Sartorelli AC. Quimioterapia del cancer. 9na ed. Madrid: Editorial Manual Moderno; 2005. p. 885-915.
44. Sasson A. Biotechnologies: Current Achievements and Prospects Social Acceptance of Biotechnology-Derived Products Medical and Pharmaceutical. Biotechnology. 2004 Jul:108-109.
45. Okamoto H, Naoki K, Narita Y. A combination chemotherapy of carboplatin and irinotecan with granulocyte colony-stimulating factor (G-CSF) support in elderly patients with small cell lung cancer. Lung Cancer. 2006;53:197-203.
46. Hernández F, García I, González CA, Valenzuela C, Soto R, Duconge' J, et al. Bioequivalence of Two Recombinant Granulocyte Colony-Stimulating Factor Formulations in Healthy Male Volunteers. Biopharm Drug Dispos. 2005;26:151-9.
47. Tsukadaira A, Okubo Y, Takashi S, Kobayashi H, Kubo K. Repeated arthralgia associated with granulocyte colony stimulating factor administration. Ann Rheum Dis. 2002;61:849-50.
48. Fukuoka M, Takada M, Masuda N, Negoro S, Kodama N, Kawahara M, et al. Dose intensive weekly chemotherapy (CT) with or without recombinant human granulocyte colony-stimulating factor (G-CSF) in extensive-stage (ES) small-cell lung cancer (SCLC). Proc Am Soc Clin Oncol. 1992;11:290.
49. Quittet P, Ceballos P, López E. Low doses of GM-CSF (molgramostim) and G-CSF (filgrastim) after cyclophosphamide (4 g/m2) enhance the peripheral blood progenitor cell harvest: results of two randomized studies including 120 patients. Bone Marrow Transplant. 2006; 38(4): 275-84. Available from: http://www.nature.com/bmt/journal/v38/n4/full/1705441a.html
50. Orozco H, Arch J, Medina-Franco H. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Archives of surgery. 2006; 141(2): 150-3. Available from: http://archsurg.ama-assn.org/cgi/content/abstract/141/2/150
51. Beveridge RA, Miller JA, Kales AN. A comparison of efficacy of sargramostim (yeast-derived RhuGM-CSF) and filgrastim (bacteria-derived RhuG-CSF) in the therapeutic setting of chemotherapy-induced myelosuppression. Cancer Invest. 1998; 16(6): 366-73. Available from:
52. Crawford J, Glaspy JA, Stoller RG. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia". Support Cancer Ther. 2005 Oct; 3(1): 36-46. Available from: http://cigjournals.metapress.com/content/x3r0272u13267758/
Recibido: 5 de octubre de 2010.
Aprobado: 13 de noviembre de 2010.
Lic. Leslie Pérez Ruiz. Center of Molecular Immunology (CIM). Street 216 and 15, Atabey, Playa. Havana City, Cuba. Email : leslie@cim.sld.cu