Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión On-line ISSN 1561-2988
Rev Cubana Farm v.45 n.2 Ciudad de la Habana abr.-jun. 2011
ARTÍCULOS ORIGINALES
Ion-exchange resin complexation: Masking the bitter taste of cefuroxime axetil
Complejación de la resina de intercambio de iones: enmascaramiento del sabor amargo de cefuroxime acetil
PhD. Inderbir SinghI; PhD. Pradeep KumarI; PhD Manju NagpalII; PhD. Sandeep AroraI
IChitkara College of Pharmacy, Chandigarh-Patiala National Patiala, Punjab, India.
IISchool of Pharmaceutical Sciences, Chitkara University, India.
ABSTRACT
OBJECTIVE: the purpose of this research was to formulate taste masked complexes of cefuroxime axetil and to evaluate them for taste, drug loading and characterized by FTIR, XRD. Tablets were formulated of selected batches and evaluated for drug release and physical parameters.
METHODS: complexation technique is used to prepare complexes of drug where ion exchange resins such as Indion® 214, Indion® 234 and Indion® 414 were used with a drug-resin ratio of 1:0.5, 1:1, 1:2. The drug resinates were characterized by Infrared Spectroscopy, DSC and X-Ray Diffraction pattern and evaluated for drug loading and taste. Direct compression method was used to formulate tablets. In vitro dissolution was carried out using USP II apparatus.
RESULT: potential taste masking increased with increasing concentration of resin. Indion® 214 resin showed better taste masking effect as compared to Indion® 234 and Indion® 414. Percent of drug loading was maximum at drug : resin ratio of 1:1, after that it decreased. Prolonged (upto 5 h) and slow drug release was observed with resin 214 at higher concentration.
CONCLUSIONS: out of three resins chosen, Indion® 214 at higher concentration exhibit excellent taste masking as well as sustained drug release action.
Key words: Cefuroxime Axetil, Taste masking, Indion® 214, Indion® 234, Indion® 414, resinate.
RESUMEN
OBJETIVO: el objetivo de esta investigación fue formular los complejos con sabor amargo de cefuroxime acetil y evaluarlos por sabor, carga medicamentosa y caracterización por FTIR, XRD. Las tabletas fueron formuladas a partir de lotes seleccionados y evaluados en busca de la liberación medicamentosa y parámetros físicos.
MÉTODOS: la técnica de complejación se utilizó para preparar complejos farmacológicos donde las resinas de intercambio iónico como Indion® 214, Indion® 234 y el Indion® 414 se emplearon a una proporción resina-medicamento de 1:0.5, 1:1, 1:2. Los resinados medicamentosos fueron caracterizados mediante espectroscopia infrarroja, DSC y el patrón de difracción-rayos-X, y evaluados para determinar la carga medicamentosa y el sabor. El método de compresión directa fue empleado para formular las tabletas. Se efectuó una disolución in vitro utilizando el equipo USP II.
RESULTADOS: el posible enmascaramiento del sabor aumentó con la creciente concentración de resina. La resina Indion® 214 mostró el mejor enmascaramiento del gusto amargo en comparación con Indion® 234 e Indion® 414. El porcentaje de carga medicamentosa fue máximo en el fármaco: proporción de la resina 1:1, después disminuyó. Se observó una liberación medicamentosa prolongada (hasta 5 h) y lenta con la resina 214 a una mayor concentración.
CONCLUSIONES: de las 3 resinas escogidas, Indion®214 a una mayor concentración muestra un excelente enmascaramiento del sabor así como una mantenida acción liberadora del fármaco.
Palabras clave: Cefuroxime acetil, enmascaramiento del sabor, Indion® 214, Indion® 234, Indion® 414, resinado.
INTRODUCTION
A number of orally administered drugs exhibit natural bitter taste that creates a burning feeling in the throat or mouth. This results in patient non-compliance to the medicines especially in case of children and elderly, thereby reducing the effectiveness of the pharmacotherapy. Therefore, it is necessary to reduce or mask the bitterness for enhancing patient acceptability and improving oral palatability of bitter tasting drugs. A number of methods have been reported for masking the bitter taste, such as use of ion-exchange resins,1 use of inclusion complexes with cyclodextrins,2 viscosity modifications3 melt granulation,4 microencapsulation techniques like spray-drying,5 spray-congealing,6 coacervation7 and solvent evaporation method.8 Ion exchange resins are solid and suitably insolubilized high molecular weight polyelectrolytes that can exchange their mobile ions of equal charge with the surrounding medium reversibly. Typically, the ionized drug and the ion-exchanger form a stable complex for the relatively short period of exposure, making the drug unavailable for taste sensation. As the formulation passes to the further parts of the GI tract, the drug is released from the ion-exchanger into the surrounding media due to low pH in the stomach, increased ionic concentration of the GI tract, larger volume of the surrounding media and/or increased gastric residence time and is, thus, available for absorption. Ion exchange resins have been widely used as a drug carrier in pharmaceutical dosage forms for their taste masking and controlled release applications.9 Cefuroxime axetil is the acetoxyethyl ester of cefuroxime and is an oral prodrug showing a bioavailability of 30 to 40 % when taken on fasting and 50 to 60 % when taken after food.10 The molecule is de-esterified during absorption, either in the mucosal cells, the portal blood, or the liver such that only cefuroxime circulates systemically. The cefuroxime axetil esterase can hydrolyze cefuroxime axetil to the nonabsorbable cefuroxime in the gut lumen and is, therefore, suspected as a possible cause of incomplete bioavailability. The absolute bioavailability of all newer oral ester prodrug cephalosporins is below 50 to 60 %, which suggests an absorption mechanism through the mucosa with limited capacity.11 The present study was aimed to prepare carboxylic acid functionalized crosslinked polyacrylic ion-exchange resins (Indion® 214, 234 and 414) based drug resinate complexes of cefuroxime axetil and to evaluate for taste and drug loading and further characterization of the complexes using Fourier Transform Infra Red (FTIR) and X-Ray Diffraction (X-RD) techniques. Drug resinates were compressed into tablets by direct compression and tablets were evaluated for hardness, friability and disintegration tests. In vitro dissolution studies were carried out to study drug release pattern from the tablet formulation.
METHODS
Cefuroxime axetil was received as gift sample from Surya Pharmaceuticals, Baddi, India, Indion® 214, Indion® 234 and Indion® 414 were kindly gifted by Ion Exchange (India) Ltd, Mumbai, India. Vivapur 102 was kindly gifted by S. Zhaveri Pharmakem Pvt. Ltd, Mumabi, India. All other chemicals/reagents were of analytical grade and were used as such.
Preparation of Drug Resin Complex
Drug resin complexes were prepared by reacting cefuroxime axetil with cation exchange resins (Indion® 214, Indion® 234 and Indion® 414) in various stoichiometric ratios (0.5:1, 1:1 and 1:2 of drug:resin). Weighed amount of resin was added to distilled water (15 mL) in a glass beaker and the suspension was stirred on magnetic stirrer for 30 min followed by the addition of the drug and the contents of the beaker were allowed to stir for another 2 h at 37 ºC. Resulting drug resinates were then separated by filtration and washed with distilled water to remove the free drug and other ions. Overnight drying of the drug resinates was carried at 40 ºC and the dried resinates were kept in a desiccator till further use.
Drug loading analysis
Dry drug resinate (50 mg) was extracted with hydrochloric acid (0.1 N, 100 mL) in a sonicating bath for 30 min. The flask was allowed to stand for 1 hour and then an aliquot (1 mL) was diluted to 10 mL with water and filtered and analyzed by UV spectrophotometry using a double beam spectrophotometer (2202, Systronics, India) at 278 nm. For each resinate type three replicate extractions were carried out.
Taste Evaluation
All the batches of drug resin complexes were subjected to gustatory sensory evaluation test performed by a panel of ten volunteers. The volunteers selected randomly and were instructed to rate the samples as per the taste evaluation scale. Same set of volunteers were employed for the taste evaluation study. Sample equivalent to 50 mg was held in mouth for 15 s. The evaluation was performed by classifying bitter taste into five classes: 0) No bitter taste, 1) Very slightly bitter taste, 2) Slightly bitter taste, 3) Appreciably bitter taste, 4) Very bitter. Significant differences among different drug resinates were analyzed using the student's unpaired t-test; a value of p< 0.05 was accepted as index of a significant difference between samples.
FTIR Spectroscopy
The drug, resin and resinate were subjected to Fourier Transform Infrared (FTIR) studies to check drug resin interaction. FTIR spectra were recorded on samples prepared in KBr using FTIR-8400S with IR solution software (Shimadzu). Data were collected over a spectral region from 4 000 to 650 cm1 with resolution of 4 cm1.
X-ray Diffraction Analysis
The samples were subjected to X-ray diffraction study for the confirmation of complex formation. X-ray powder diffraction patterns were recorded on an X-ray diffractometer (Model X»Pert, Philips, Netherlands) using Ni-filtered, Cu K radiation, voltage of 40 kV and 25 mA current. The scanning rate employed was 1° min1 over the 0-100° diffraction angle (2q) range.
Formulation and evaluation of tablets
The tablets of taste masked resinates of cefuroxime axetil were prepared by direct compression using single stroke multipunch tableting machine (AK Industries, Nakodar, India). The tablet weight was kept 250 mg for all the batches consisting of resinate equivalent to 50 mg of drug. The formulated tablets were evaluated for hardness, friability and disintegration time.
Hardness: Ten tablets from each batch were examined using Monsanto hardness tester.
Friability: Ten tablets were weighed (W1) and rotated at one hundred revolutions for 4 minutes in a Roche friabilator. The tablets were then reweighed (W2) and the percentage weight loss (% F) was calculated:
% F= W1 - W2 / W1 x 100
Disintegration time: Six tablets from each batch were examined in USP disintegration test apparatus.
In vitro dissolution
A USP 23 dissolution test apparatus II (paddle type) at 37 ± 0.5 °C and 50 rpm using 900 mL of 0.1 N HCl (pH 1.2) as dissolution medium was used to characterize the release of cefuroxime axetil from the tablets (selected batches) formulated using drug resinates. Aliquots of 5 mL were withdrawn at predetermined time intervals and an equal amount of fresh dissolution medium was added. Test samples were filtered through whatman filter paper No. 41 (Whatman Paper Limited, UK), suitably diluted and assayed at 278 nm using a blank solution as reference with a UV-Vis double-beam spectrophotometer (Systronics 2202, India). The cumulative percentage of cefuroxime axetil dissolved was calculated using a regression equation generated from the standard data.
RESULTS
Taste evaluation
A panel of 10 members using time intensity method was employed for discriminating (in terms of bitterness) various batches of resinates formulated using different ion exchange resins in different proportions. The taste masking potential of the selected resins was found to increase with increasing concentration of resin in drug resinate. Indion® 214 exhibit better taste masking potential at all ratios as compared to Indion® 234 and 414 as indicated by bitterness score. However Indion® 234 and 414 resins showed taste masking only at higher concentration. The order of taste masking potential of different resins is Indion® 214> Indion® 234> Indion® 414 (table).
Drug Loading Analysis
Drug loading was found to increase with increasing fraction of resin but it is upto some extent. 1:1 ratio (batch F214II, F234II and F414II) showed maximum loading after that decrease in drug loading was observed (table). Indion® 214 and 414 resins showed higher increase in drug loading on increasing concentration as compared to that with Indion® 234 resin.
FTIR Spectroscopy
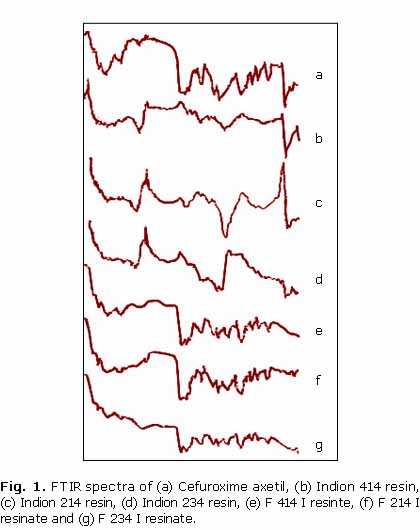
The FTIR spectra of pure cefuroxime axetil showed characteristic peaks at 1724.24 cm1 (C= O stretching) together with peaks at 1070.42 cm1 and 3492.85 cm1characterizing C-O stretching of ester group and N-H stretching of amide groups respectively. The peak at 1328.86 cm1 indicated the presence of aromatic ether in the drug moiety. Significant reduction in the intensity of distinctive peaks of drug demonstrates the formation of complex between drug and the resin molecule (fig. 1).
X-Ray Diffraction
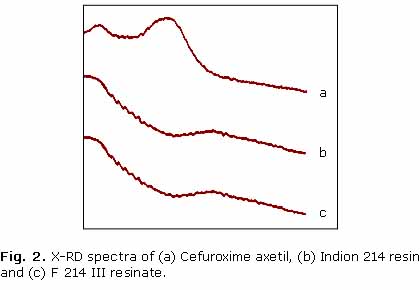
Characteristic peaks appeared in the X-RD pattern of the drug suggested about the crystalline nature of the drug. Low intensity of cefuroxime axetil peaks in drug resinate suggested that the drug in the complex is converted into amorphous form (fig. 2).
Evaluation of tablets
Parametric evaluation:
Friability of the tablets was found to be between 0.60 to 0.80 % indicating good mechanical resistance of the formulated tablets of drug resinates. Hardness was found to be between 2-4 kg/cm2. The disintegration time of tablets was found to be between 1-2 min. As no disintegrating agent was included for formulating tablets, the effective tablet disintegration was due to extensive swelling properties of the selected resins.
In vitro dissolution
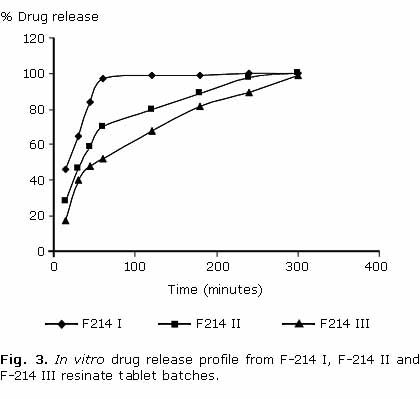
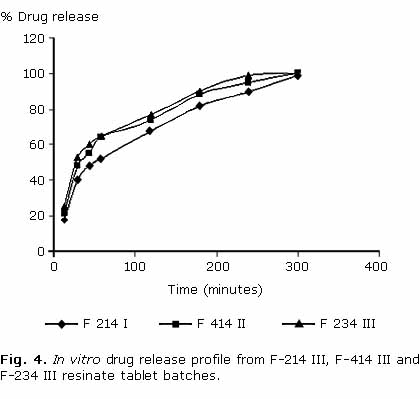
In vitro drug dissolution rate from different batches (F-214 I, F-214 II, F-214 III) containing Indion® 214 was found to be decreased with increasing concentration of resin with in 60 min i.e. F-214 I> F-214 II> F-214 III. Almost 100 % drug release with F-214 I, about 70 % with F-214 II and about 50 % with F-214 III was achieved with in first 60 min. However extent of drug release was same with three batches and sustained release up to 5 h (100 %) was observed (fig. 3). Further in vitro dissolution of batch F214 III was compared with batches F-234 III and F-414 III. Dissolution rate from these batches follows the order: F-234 III> F-414 III> F-214 III. However there is no significant difference in dissolution rate among different batches. Sustained and complete drug release up to 5 h was observed with all batches (fig. 4).
DISCUSSION
Complexation of cefuroxime axetil with cation exchange resins (Indion® 214, 234 and 414) was found to increase the acceptability and palatability of the drug. However, 1:2 ratio of drug resin complex of Indion® 214 was found to exhibit better taste masking properties compared to drug resinates of Indion® 234 and 414. The taste masking ability of different resins exhibit concentration dependence as it improves at higher concentrations. Higher concentration may lead to interaction of each drug particle within resin network thereby improving taste masking by the resins. The bitterness score suggested Indion 214 as the best one as compared to Indion 234 and Indion 414. the drug loading was found to be increased with increasing fraction of resin but upto 1:1 ratio. The decreased drug loading after that may be due to increased viscosity of the drug-resin mixture. Characterization of drug-resin complex via FTIR and X-RD further confirms the interaction between drug particles with resin network thereby providing taste masked drug particles. Taste masked tablet batches pass the physicochemical evaluation tests suggesting that the formulation complies as per official standards. Along with taste masking drug resin complex also exhibits sustained release characteristics with increasing fraction of resin. However different resins showed same release rate at higher ratios with complete drug release with in 5 h. This suggested that type of resin does not affect the release characteristics at higher drug resin ratios.
Acknowledgement
The authors are grateful to Dr. Madhu Chitkara, Director, Chitkara Institute of Engineering and Technology, Rajpura, Patiala, India and Dr. Ashok Chitkara, Chairman, Chitkara Educational Trust, Chandigarh, India for support and institutional facilities.
REFERENCES
1. Agarwal R, Mittal R, Singh A. Studies of Ion-Exchange Resin Complex of Chloroquine Phosphate. Drug Dev Ind Pharm. 2000;26(7):773-6.
2. Patel AR, Vavia PR. Preparation and evaluation of taste masked famotidine formulation using drug/beta-cyclodextrin/polymer ternary complexation approach. AAPS PharmSci Tech. 2008;9(2):544-50.
3. Blase CM, Shah MN. Aqueous pharmaceutical suspensions for pharmaceutical actives. United States Patent 5409907.1995.
4. Appelgren C, Eskilson C. Novel method for the granulation and coating of pharmacologically active substance. Drug Dev Ind Pharm. 1990;16:2345-51.
5. Bora D, Borude P, Bhise K. Taste Masking by Spray-Drying Technique. AAPS PharmSci Tech. 2008;9(4):1159-64.
6. Yajima T, Nogata A, Demachi M, Umeki N, Itai S, Yunoki N, et al. Particle design for taste-masking using a spray-congealing technique. Chem Pharm Bull. 1996;44(1):187-91.
7. Al-Omranm MF, Al-Suwayeh SA, El-Helw AM, Saleh SI. Taste masking of diclofenac sodium using microencapsulation. J Microencapsul. 2002;19(1):45-52.
8. Shidhaye S, Malke S, Kadam V. Taste masked, orally disintegrating tablet containing microspheres for immediate release. J Pharm Res. 2008;1:225-9.
9. Singh I, Rehni AK, Kalra R, Joshi G, Kumar M, Aboul-Enein HY. Ion Exchange Resins: Drug Delivery and Therapeutic Applications. FABAD J Pharm Sci. 2007;32:91-100.
10. Finn A, Straugun A, Meyer M, Chubb J. Effect of dose and food on the bioavailability of cefuroxime axetil. Biopharm Drug Dispos. 1987;8:519-26.
11. Kees F, Lukassek U, Naber KG, Grobecker H. Comparative investigations on the bioavailability of cefuroxime axetil. Arzneimittelforschung. 1991;41:84.3.
Recibido: 5 de octubre de 2010.
Aprobado: 13 de noviembre de 2010.
PhD. Inderbir Singh. Chitkara College of Pharmacy, Chandigarh-Patiala National Highway, Rajpura-140401, Patiala, Punjab, India. E-mail: inderbirsingh2906@gmail.com