Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión impresa ISSN 0034-7515versión On-line ISSN 1561-2988
Rev Cubana Farm v.45 n.2 Ciudad de la Habana abr.-jun. 2011
PRODUCTOS NATURALES
Fungicidal activity of Eucalyptus tereticornis essential oil on the pathogenic fungus Fusarium oxysporum
Actividad antimicótica del aceite esencial a partir de Eucalyptus tereticornis sobre el hongo patógeno Fusarium oxysporum
Walter Murillo Arango; José Miguel Acevedo Ruíz; Carlos Alberto Peláez Jaramillo
Grupo Interdisciplinario de Estudios Moleculares (GIEM), Universidad de Antioquia. Medellín, Colombia.
ABSTRACT
The objective of present paper was to determine the antifungal activity of the Eucalyptus tereticornis (Myrtaceae) essential oil and two fractions on the Fusarium oxysporum mushroom, a pathogen with clinical and agricultural significance. The total citronelal (44.8 %) and geraniol (9.78 %) essential oil had a fungicidal effect at a 3 g/L concentration and a fungicidal activity at small concentrations. The A and B fractions composed most of p-mentane-3,8-diol (18.95 %) and geraniol acetate (24.34 %), respectively were more active than the total extract. The observations at microscopic level showed damages and changes in hyphae and chlamydospores, as well as a decrease in the number of conidia. The observed fungicidal activity and the morphologic damages were dependent on the concentration.
Key words: Eucalyptus tereticornis, Fusarium oxysporum, fungicidal activity, essential oils.
RESUMEN
El objetivo de este trabajo fue determinar la actividad antifúngica del aceite esencial de Eucalyptus tereticornis (Myrtaceae) y 2 fracciones sobre el hongo Fusarium oxysporum, patógeno de importancia tanto clínica como agrícola. El aceite esencial total, compuesto principalmente por citronelal (44,8 %), citronelol (9,78 %) presentó un efecto fungicida a una concentración de 3 g/L y actividad fungistática a concentraciones menores. La fracciones A y B compuestas en su mayoría por p-mentano-3,8-diol (18,95 %) y acetato de citronelol (24,34 %) respectivamente fueron más activas que el extracto total. Las observaciones a nivel microscópico mostraron daños y cambios en hifas y clamidosporas, así como disminución en el número de conidias. La actividad fungistática observada y los daños morfológicos fueron dependientes de la concentración.
Palabras clave: Eucalyptus tereticornis, Fusarium oxysporum, actividad fungicida, aceites esenciales.
INTRODUCTION
Various species of Fusarium have been reported to affect wheat plantations causing contamination due to the production of mycotoxins, becoming a risk factor in animals and humans1 and causing considerable financial losses.2,3 Over the past years, there has been an increase in their appearance in human infections, and is currently considered the second invasive infection-causing fungus in patients with immunosuppression, and is associated with high rates of morbility and mortality.4,5 The genus contains 100 species of which the most known as pathogens are Fusarium solani and Fusarium oxysporum. However, there are other species that have been reported as plant, animal, and human pathogens.6 The Fusarium species are resistant in vitro to the majority of fungicides approved for the mycosis treatment in humans such as those of the azole and polyene types, which are the ones most commonly used.7 Large quantities of essential oils are known for exerting antifungal activity.8-11 The aim of this study was to determine the fungicidal activity of the oil by morphological assessment of damage caused and the effect of total oil composition on the biological activity.
METHODS
Essential oils Extraction
Eucalyptus tereticornis (Myrtaceae) leaves collected from a plant located in the Universidad de Antioquia (Medellin), city with a humid subtropical climate, temperature between 16 and 28 °C. The specie was identified and kept in the Universidad de Antioquia Herbarium with the register number HUA 2923. The essential oil were obtained by hydro distillation of the fresh material. The oil collected was dried over anhydro sodium sulphate resulting in a yellow, translucid liquid with a density of 0.855 g/mL, with a yield of 0.89 %.
Fractionation of essential oil by column chromatography
Five grams of the total extract of the essential oil from E. tereticornis were weighed and separated into a column packed with silica gel 60 F using benzene/ethyl acetate as an elution system in a proportion of v/v 98:2 with progressive increment of the ethyl acetate quantity until obtaining a proportion of 10 % with respect to the quantity of benzene used at the beginning of the separation.
GC/MS analysis of the essential oil and its fractions
The essential oil and its principal fractions obtained by column chromatography were analyzed by gas chromatography GC (Agilent 6890) by injecting 1 µL of solutions with a concentration of 1 g/L in hexane for each of the samples analyzed in a HP5 MS column (cross linked 5 % PH ME siloxane) with dimensions of 30 m x 0.25 mm X 0.25 µm. The starting temperature was 60 °C for 10 min, then 250 °C for 15 min, and finally 280 °C for 10 min in the post run; electronic impact at 70 ev. The quantification of the components was expressed as relative percentages of the total area of the chromatograms.
The identification of the components of the essential oil and its fractions was based on the patterns of fragmentation obtained in their mass spectra in comparison to the mass spectra of the databases of Wiley or NIST 2005.
Fugal strain
The fungus used in the evaluation of the fungicide activity was Fusarium oxysporum, identified in the Center of Microbiological Research (CIMIC) of the Universidad de Los Andes in Bogotá (Colombia) and kept in the microbiology laboratory of the Grupo Interdisciplinario de Estudios Moleculares of the Universidad de Antioquia.
Antifungal Assay
Potato dextrose agar (PDA) was the culture medium used. Essential oil concentrations were prepared at 0.01, 0.1, 1, 2, 3, and 4 g/L, in a volume of 13 mL of agar, adding the corresponding amounts of each in test tubes with the sterile medium, melted and cooled to 45 ºC. Each tube was placed in a vortex at 3000 r·m-1 to homogenize the mixture of agar and oil. The mixture was poured into sterile petri dishes until the medium had solidified. A 0.5 cm-diameter slice of fungus mycelium was planted and incubated at room temperature for 10 days (until the fungus corresponding to the control reached the full petri dish diameter). Culture medium PDA without extract was used as the blank, and the commercial fungicide Dithane FMB was used as the positive control at the concentration recommended by the manufacturers (10 g/L).
A growth kinetic was followed by evaluating the diameter every 24 h for approximately 10 days. The inhibition percentages were calculated with the following algorithm.
Inhibition %= [dc - dt]/dc* 100
dc= colony diameter in the control (cm) and dt= colony diameter in the treatment (cm). Three replicas were made for each treatment.
Effects on the fungus structures
The morphological effects caused by the oil were determined by the comparative analysis of the structures observed in the control and in each of the treatments under an optical microscope model BX50 Olympus® at (1 000 X).
Conidia recount
An area of 0.5 cm of the culture medium was taken where the mycelium growth was uniform both for the control and each of the treatments. The mycelium was removed and placed in a test tube containing 1 mL of peptonated water. A drop of tween 20 was added to each test tube and was sonicated for 30 min to ensure the release of the spores and conidia. Subsequently, 10 µL were taken from each tube and the number of spores was recounted in the Neubauer chamber.
Statistical analysis
CI50 and CMI were determined by means of regression analysis using Statgraphics plus, version 5.1.
RESULTS
Composition analysis by GC/MS
The composition of the extract and of fractions A and B is shown on table, which shows that main components of the total extract correspond to citronellal, citronellol, citronellic acid, and linalool epoxide, whereas for fraction A p-menthane-3,8-diol, caryophyllene oxide and 2,2-dimethyl-5-(1-methyl-ethyl)-tetrahydrofuran stood out. Citronellic acid and linalool epoxide stood out in fraction B.
Table. Identification and relative composition of E. tereticornis and its fractions
(A and B)
| No. | Compound | E. tereticornis | Fraction A | Fraction B |
| 1 | Pinene | 1.58 | - | - |
| 2 | 1.8 cineole | 3.10 | - | - |
| 3 | 2,6- dimethyl-5-Heptenal, | 2.44 | - | - |
| 4 | Citronellal | 44.8 | - | 1,46 |
| 5 | Citronellol | 9.78 | - | 1.89 |
| 6 | Epoxy-linalooloxide | 7.59 | 1.65 | 6.27 |
| 7 | Citronellic acid | 6.47 | - | 9.14 |
| 8 | Citronellol acetate | 1,06 | - | 24.34 |
| 9 | p-menthane-3,8-diol | 1.0 | 18.95 | 1.08 |
| 10 | Caryophyllene oxide | 1.26 | 5.11 | - |
| 11 | No ident | 2.0 | 8.20 | - |
| 12 | No ident | 1.06 | 2.93 | - |
| 13 | No ident | 1.16 | - | |
| 14 | 2,6-dimethyl-6-Hepten-2-ol | 2.57 | - | |
| 15 | No ident | + | 2.06 | 2,46 |
| 16 | 2,2-dimethyl-5-(1-methylethyl) tetrahydrofuran | 1.61 | 9.47 | 5.05 |
| 17 | Myrcenol | - | 8.20 | - |
| 18 | Isomentone | * | 6.79 | - |
| 19 | 5-methyl-2-(1-methylethyl) Cyclohexanone | 1.26 | 2.58 | - |
| 20 | Citronellol epoxide | * | 5.84 | - |
| 21 | 5,9-dimethyl-1-decanol | * | 2.2 | |
| 22 | No ident | * | 1.36 | - |
| 23 | Hidroxi citronellal | * | 2.41 | 1.47 |
| 24 | Citronellyl isobutyrate | +/- | - | 1.17 |
| 25 | No ident | * | - | 1.97 |
| 26 | No ident | * | - | 5.42 |
| 27 | No ident | * | - | 2.46 |
| 28 | No ident | * | - | 2.76 |
*Peak that has a total area percentage inferior to 1%; +/-: peak
partially identified with a maximum percentage of 10 % of the total area of
compounds identified; -: compound absent in the sample analyzed.
Fungicidal activity of the essential oil from E. tereticornis and fractions on F. oxysporum
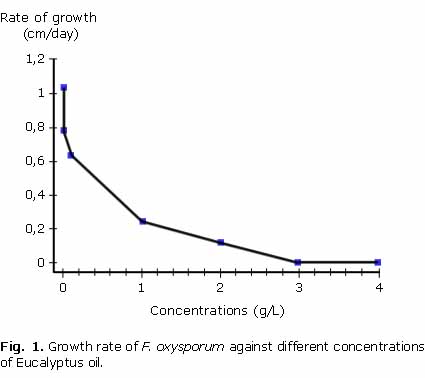
The results of the inhibiting effect of the oil are presented on figure 1, on which the speed of fungus growth is shown as a function of total oil concentration. Each point on the graph represents the value of the slope obtained from the growth curves for each concentration and shows how the speed of growth decreases as oil concentration increases, reaching a very low rate (0.12 cm/day) at 2 g/L and becomes zero when the concentration is near 3 g/L. The control presented a growth rate of 1.03 cm/day. The essential oil from E. tereticornis had a greater inhibiting activity than the Dithane FMB fungicide used at the concentration recommended by the manufacturers (1 % w/w) and which is equivalent to 10 g/L; at this concentration of commercial product the fungus grows at 0.42 cm/day, while at an oil concentration 10 times lower (1 g/L), the fungus grows at 0.23 cm/day demonstrating an activity almost two times greater than the commercial fungicide.
The calculated values for the 50 % inhibitory and minimum inhibitory concentrations were IC50= 0.6718 g/L MIC= 2.687 g/ L, respectively.
These were obtained with the equation adjusted for the model of regression: % Inhibition= 1.73(concentration)1/2 r2= 99.7 and P-value= 0.0041.The fungicide activity of fractions A and B was evaluated at two concentrations. The respective inhibition percentages at 0.5 g/L were of 50.8 and 76.15 %, while at 1 g/L both fractions revealed a total inhibition of fungus growth.
Effects on the fungus structures
The effects on fungus morphology by the oil at concentrations of 0.01, 0.1,1 and 2 g/L can be observed in greater detail at microscopic level.
The most notorious effects, observed under optical microscope, were the damage to hyphae, which were affected at an oil concentration of 2 g/L, figure 2 (a and f). The effects on fungus reproductive structures such as conidia and macroconidia showed an inverse correlation with the essential oil concentration.
Conidia recount
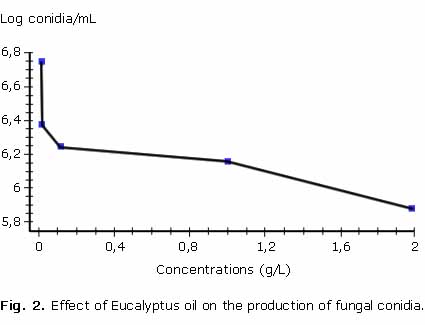
Conidia recount in each of the treatments revealed a decrease in number with respect to the control. The values are shown on figure 3. The effects on fungus reproductive structures such as conidia showed an inverse correlation with the essential oil concentration since its production significantly decreased as the concentration increased. A decrease in chlamydospores, structures that offer fungi resistance under unfavorable conditions.
DISCUSION
The fungistatic activity shown by the essential oil extract from E. tereticornis at concentrations inferior to 3 g/L was evident when observed under a microscope. Effects on fungus morphology are observed, especially on hyphae. This explains the reduction in mycelium growth as well as its depigmentation. Similar studies have reported the effect of essential oils against fungi such as Aspergillus niger and A. parasiticus12 where there are irreversible effects caused (on a structural level) on wall, membrane, and hyphae, as well as on the production of some damaging metabolites or mycotoxins such as aflatoxins.13
This study shows the morphology alterations caused by E. tereticornis essential oil on the pathogenic fungus Fusarium oxysporum at the level of cell structures. The most notorious effects, observed under optical microscope, were the damage to hyphae, which were affected at an oil concentration of 2 g/L. In the same manner, the effects on fungus reproductive structures such as conidia and macroconidia showed an inverse correlation with the total essential oil concentration. A decrease in chlamydospores, structures that offer fungi resistance under unfavorable conditions, was also evidenced in the microscope observations.
Despite the differences in the composition of essential oil of E. tereticornis shown in a number of reports,14,15 is common in them to find compounds such as 1.8 cineol, p-cymene and p-menthane-3 ,8-diol, some also found in our study.
The increment in fungicide activity when carrying out the chromatographic separation showed that the composition of the mixture is an important factor in the activity, this has been discussed in similar studies on phytopathogenic fungi16 given that the type of compound and its concentration determines the level of the activity and certain components of the mixture or its combination could imply a synergistic behavior in the biological activity.
It is important to note that both fractions are more active than the total extract. Fraction B shares eight of its components with the total extract (4, 5, 9, 25, 26, 27, 28, and 29) with a notable increase in the percentage of citronellol acetate with respect to total extract; this fraction was also the one of greatest fungicide activity. Although it cannot be discarded that the diverse components present in the total extract participate in the fungicide activity, the notable activity increase of the fractions and their closeness in composition may indicate that the common components have a greater weight in the fungicide activity.
This evidence supports the possibility of using essential eucalyptus oils for the control of pathogenic fungi in medical and agricultural contexts.
CONCLUSION
In this report we provide evidence on the mechanism of antifungal action of essential oil of E. tereticornis on the pathogenic fungus F. oxysporum
ACKNOWLEDGEMENT
We express our thanks to the mycology laboratory at the Universidad de Antioquia and to Jesus Laraondo of ANDERCOL S.A for your cooperation in this work.
REFERENCES
1. Prandini A, Sigolo AL, Filippi AP, Battilani B, Piva G. Review of predictive models for Fusarium head blight and related mycotoxin contamination in wheat Food. Chem Toxicol. 2009;47:927-31.
2. Ruiz-Roldán MC, Di Pietro A, Huertas-González MD, Roncero MIG. Two xylanase genes of the vascular wilt pathogen Fusarium oxysporum are differentially expressed during infection of tomato plants. Mol Gen Genet. 1999;261:530-6.
3. Alves-Santos FM, Cordeiro-Rodrigues L, Sayagués JM, Martín-Domínguez R, García-Benavides P, Crespo MC, et al. Pathogenicity and race characterization of Fusarium oxysporum f.sp. phaseoli isolates from Spain and Greece. Plant Pathol. 2002;51:605-11.
4. Izquierdo AA, Cuenca EM, Monzon A, Mellado E, Rodríguez T. Antifungal susceptibility profile of clinical Fusarium spp. Isolates identified by molecular methods. J Antimicrobial Chemother. 2008;61:805-9.
5. Fonseca-Nogueira II, Pereira RN, De Queiroz LA, Correia-Magalhães OM, Massa-Lima DM. Fusarium lateritium (nees) as an Agent of Fungemia in a Patient Infected with the Human Immunodeficiency Virus (HIV) Brazilian. J Microbiol. 2007;38:285-6.
6. Nelson PE, Dignani MC, Anaissie EJ. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479-504.
7. Cuenca EM, Gomez LA, Mellado E. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother. 2006;50:917-21.
8. Worth JL, Slavin AM. Bloodstream infections in haematology: Risks and new Challenges for Prevention. Blood Rev. 2009;23:113-22.
9. Ramezani S, Batish K. Antifungal Activity of the Volatile oil of Eucalyptus citriodora. Fitoterapia. 2002;(73):261-2.
10. Murillo E, Villa A, Linares A. Composición química, actividad insecticida y fungicida de Ocimum micranthum, Rev Colombiana Entomol. 2002;28(1):109-13.
11. Barreto AG, Velázquez P, Peña M, Rodríguez TH. Evaluación in vitro de extractos de Eucalyptus citriodora hook y Eucalyptus saligna sm como posibles antisépticos mamarios. Rev Producción Animal. 2006;18(2):35-40.
12. Sharma N, Tripathi A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol Res. 2008;163:337-344.
13. Rasoolia I, Bagher, RM, Allameh A. Growth inhibition and morphological alterations of Aspergillus niger by essential oils from Thymus eriocalyx and Thymus x-porlock. Food Control 2006;17:359-64.
14. Alitonou G, Avlessi F, Wotto VD, Ahoussi E, Dangou J, Dominique CK, et al. Composition chimique, proprietés antimicrobiennes et activités sur les tiques de l'huile essentielle d'Eucalyptus tereticornis Sm. C. R. Chimie. 2004;7:1051-5.
15. Batish DR, Singh HP, Kohli RK, Kaur S. Eucalyptus essential oil as a natural pesticide. Forest Ecol Man. 2008;256:2166-74.
16. Lee YS, Kim J, Lee SG, Oh E, Shin SC, Park IK. Effects of plant essential oils and components from Oriental sweetgum (Liquidambar orientalis) on growth and morphogenesis of three phytopathogenic fungi. Pesticide Biochem Physiol. 2009;93:138-43.
Recibido: 30 de noviembre de 2010.
Aprobado: 9 de enero de 2011.
Walter Murillo Arango. Grupo Interdisciplinario de Estudios Moleculares (GIEM), Universidad de Antioquia. P.O. Box 1226, Medellín, Colombia. E-mail: wmurillo@ut.edu.co