Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión impresa ISSN 0034-7515
Rev Cubana Farm vol.46 no.2 Ciudad de la Habana abr.-jun. 2012
PRODUCTO NATURAL
Effects of D-002 on aspirin-induced ulcers and neutrophil infiltration on the gastric mucosa
Efecto del D-002 sobre la úlcera inducida por aspirina asociada al infiltrado de neutrófilos en la mucosa gástrica
MSc. Maikel Valle Clara, Dra. C. Miriam Noa Puig, Dra. C. Sarahí Mendoza Castaño, MSc. Ambar Oyarzábal Yera, Dra. C. Vivian Molina Cuevas, Téc. Nilda Mendoza Hernández, Dra. C. Rosa Mas Ferreiro
Centro de Productos Naturales, Centro Nacional de Investigaciones Científicas (CNIC). La Habana, Cuba.
ABSTRACT
Introduction: the gastric mucosa is susceptible to the effects of aggressive factors, which are counterbalanced by mucosal defensive factors. Acid peptic diseases result from the imbalance between these aggressive and defensive factors. Aspirin-induced ulcer is a model of NSAIDs-induced damage in which neutrophil infiltration plays a key role.
Objective: this paper investigates the protective effect of D-002 against aspirin-induced ulcers and associated neutrophil infiltration in the gastric mucosa.
Methods: rats were randomized into six groups of 8 rats each. A negative vehicle control, and five aspirin (300 mg/kg)-treated groups: a positive control, orally treated with the vehicle, three with D-002 (25, 50 and 100 mg/kg, respectively) and other with 10 mg/kg Omeprazole. Five hours after induced damage the rats were sacrificed. The stomachs were removed and opened, and lesions examined macroscopically and microscopically. Ulcer indexes and neutrophil infiltration per ulcer areas were measured.
Results: all positive, none negative, controls exhibited aspirin-induced ulcers. Oral treatment with D-002 (25-100 mg/kg) dose-dependently and significantly reduced aspirin-induced gastric lesions (37 to 75 %), the mean number of microscopic ulcers (40 to 72 %) and neutrophil infiltration (41.7 to 83.1 %) in the rat gastric mucosa.
Conclusion: Oral treatment with D-002 (25-100 mg/kg) effectively protects against aspirin-induced ulcers and decreases the neutrophil infiltration in the gastric mucosa induced by aspirin ulceration.
Key words: D-002, gastric ulcer, neutrophil infiltration, ASA, rats.
RESUMEN
Introducción: la integridad de la mucosa gástrica depende del balance entre los factores agresivos y defensivos. La úlcera inducida por aspirina es un modelo de daño por antiinflamatorios no esteroidales en el cual el infiltrado de neutrófilos desempeña una función fundamental.
Objetivo: evaluar el efecto protector del D-002 sobre la úlcera inducida por aspirina asociada al infiltrado de neutrófilos en la mucosa gástrica.
Métodos: las ratas fueron aleatorizadas en seis grupos de ocho animales cada uno. Un control negativo con vehículo y cinco grupos tratados con aspirina (300 mg/kg): un control positivo, tratado por vía oral con vehículo, tres grupos con D-002 (25, 50 and 100 mg/kg respectivamente) y otro con omeprazol 10 mg/kg. Cinco horas después de inducido el daño las ratas fueron sacrificadas y se extrajeron sus estómagos para su análisis morfológico. Se determinó el índice de úlcera, el número de úlceras microscópicas y el número de neutrófilos por área ulcerada.
Resultados: todos los controles positivos y ninguno negativo mostraron lesiones en la mucosa. El tratamiento por vía oral con D-002 (25-100 mg/kg) redujo de modo significativo y dependiente de la dosis el índice de úlceras gástricas (37-75 %), el promedio de úlceras microscópicas (40-
72 %) y la infiltración de neutrófilos (41,7-83,1 %) en la mucosa de las ratas.
Conclusiones: el tratamiento por vía oral con D-002 (25-100 mg/kg) protege la mucosa gástrica de las ratas del daño inducido por aspirina, lo que disminuye el índice de úlcera y el infiltrado de neutrófilos.
Palabras clave: D-002, úlcera gástrica, infiltración de neutrófilos, aspirina, ratas.
INTRODUCTION
Gastric mucosa damage results from the imbalance between aggressive factors (acid, pepsin, H. pylori, non steroidal anti-inflammatory drugs (NSAIDs) and mucosal defensive factors (bicarbonate, mucus secretion, blood flow, cellular regeneration, prostaglandins (PG) and growth factors.1-5
The gastric mucosa produces arachidonic acid (AA)-derived metabolites via cicloxygenase (COX) and lipoxygenase (LOX) pathways, like PG and leukotrienes (LTs) which exhibit cytoprotective and pro-ulcerogenic properties, respectively. NSAIDs block COX, thus inhibiting the production of cytoprotective PG, which in turn lowers mucus and bicarbonate secretion, mucosal blood flow, produces neutrophil infiltration, alters the microvascular structures and increases acid and pepsinogen secretion, all of which leads to develop NSAIDs-induced ulcers. Also, the NSAIDs-induced COX inhibition shuttles the AA metabolism towards the overproduction of noxious 5-LOX derived metabolites, like LTs, hydroperoxyeicosatetranoic acids (HPETEs) and derived reactive oxygen species (ROS).3,6-8
Increased generation of ROS (superoxide anions and hydroxyl radicals) by NSAIDs, together with PG suppression, leads to microvessel occlusion and overproduction of ROS metabolites.3,6-10 Xantine-xantine oxydase system and the polimorphonuclear leukocytes (neutrophils) represent the two main sources of ROS in the gastric mucosa.11,12
Aspirin, the most widely consumed NSAID,13 induces gastric lesions due to the irreversible and non-selective inhibition of COX. It has been demonstrated that increased production of ROS, increased lipid peroxidation and neutrophil infiltration play a critical role in the pathogenesis of aspirin-induced ulcers.14-17
D-002, a mixture of 6 higher aliphatic alcohols wherein triacontanol is the most abundant, has been shown to produce gastroprotective effects.18-25 Experimental evidences support that the gastroprotective effect of D-002 involves increased gastric mucus secretion, improved mucus composition19,20 and reduced lipid peroxidation21 in the rat gastric mucosa. On the other hand, D-002 treatment has been shown to reduce acetic acid-induced colitis, and particularly, the neutrophil infiltration into the colon wall.26,27 No previous study, however, had investigated its effects on NSAIDs-induced gastric neutrophil infiltration.
Keeping in mind this background, this study was aimed to carry research into the protective effect of D-002 against neutrophil infiltration in the gastric mucosa of rats with aspirin-induced ulcers.
METHODS
Animals
Sprague Dawley rats (200-220 g), purchased in the National Centre for Laboratory Animal Production (CENPALAB, Havana, Cuba) were adapted for 7 days to the experimental conditions: temperature 25 ± 2 oC, humidity 60 ± 5 % and light/dark cycles of 12 h. During this period, the rats had free access to tap water and standard chow (rodent pellets from CENPALAB). Prior to ulcer induction, however, rats were fasted for 24 h, with water ad libitum.
Experiments were conducted in accordance to the Cuban guidelines of Animal Handling and the Cuban Code of Good Laboratory Practices (GLP). Study protocol and animal use were approved prior to study by an independent animal ethics committee.
Dosage and administration
Aspirin and omeprazole were supplied by the Cuban Medical Pharmaceutical Industry (Havana, Cuba).
The D-002 batch, supplied by the Plants of Natural Products (National Centre for Scientific Research, Havana, Cuba), had the following composition: triacontanol, 26.6 %; octacosanol, 17.5 %, dotriacontanol, 17.0 %; hexacosanol, 15.3 %, tetracosanol, 13.2 % and tetratriacontanol, 2.2 % (purity 91.8 %).
D-002 and omeprazole were suspended in a 2 % Tween 20/water vehicle (10 mg/100 mL). Aspirin was dissolved (50 mg/ml) in a 1 % acacia gum solution. All solutions and suspensions were prepared two hours prior to use, after verifying their stability and homogeneity.
Rats were randomized into six groups of 8 rats each. A negative vehicle control, and five groups that received aspirin (300 mg/kg),28 for ulcer induction: a positive control, treated orally with the vehicle, three treated with D-002 (25, 50 and 100 mg/kg, respectively) and the other with omeprazole 10 mg/kg. Treatments were given by oral gastric gavage (5 mL/kg of bodyweight) for seven days prior to ulcer induction.
Induction of ulcers by aspirin
After the last dosing rats were fasted for 24 h and then orally administered a single dose of the vehicle (negative control) or aspirin 300 mg/kg (all other groups). Five hours later the rats were sacrificed under ether anesthesia.29
Assessment of ulcer damage and histopathological findings
After the sacrifice, rat stomachs were immediately removed, opened along the greater curvature and washed with saline solution. The lesions were examined macroscopically under a microscope (magnification 3x) by two independent blinded observers. The ulcer indexes, the number of microscopic ulcers and neutrophils per area of microscope field were quantified.
The ulcer indexes corresponded to sum of the lengths of the gastric lesions (in mm). The protective ratio as the inhibition (%) according to the following formula: [Inhibition (%)= (a - b)/a x 100], wherein a was the ulcer index of the positive control group and b the ulcer index of the test group.
Samples of the ulcer areas together with the surrounding areas were taken and fixed in 10 % neutral formaldehyde (BDH, Poole, Dorset, England), dehydrated in reagent degree ethanol, embedded in paraffin, and cut in 4 µm transverse sections that were stained with haematoxylin and eosin (Merck, Darmstadt, Germany), and were examined (10 images per animal) using an Olympus BH2 microscope.
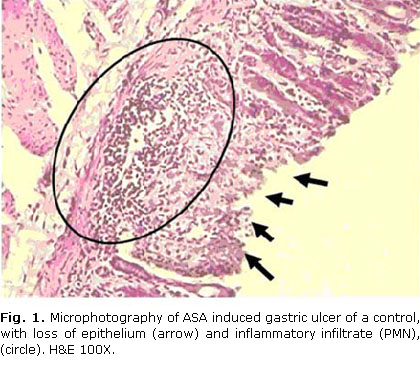
Ulcer lesions were identified as epithelium erosions accompanied by inflammatory infiltrates.
Neutrophils were counted in each complete cross-section of mucosa in accordance to Nygard et al. (1994).30 Mean neutrophil counts were determined in three sections per animal per group and afterwards averaged for each group.
Statistical analyses
Comparisons among the groups were done with the Kruskal Wallis test, paired comparisons between control and treated groups with the Mann-Whitney U test. Dose-relationships were explored with a linear regression analysis. Statistical significance was chosen for a= 0.05. Data were processed with the statistical software Statistics for Window (Release 6.1 Stat Soft Inc, Tulsa OK, USA).
RESULTS
Effects on aspirin-induced ulcers
All the positive controls, but none negative control rats, exhibited aspirin-induced erosive ulcers surrounded of inflammatory infiltrates. Figures 1 y 2 illustrate the observed lesions.
Oral treatment with D-002 dose-dependently (R= 0.98) prevented aspirin-induced gastric ulcers. D-002 given at 50 and 100 mg/kg, not at 25 mg/kg, significantly (p< 0.001) reduced ulcer indexes (70 and 75 %, respectively) as compared to the positive control group (table 1). The positive control group averaged 8 microscopic ulcers per rat, a variable significantly reduced by D-002 in a dose-dependent manner (R= 0.97). The doses of 50 and 100 mg/kg, not 25 mg/kg, reduced the average number of these lesions by 62.5 and 71.9 %, respectively. Omeprazole 10 mg/kg reduced the ulcers indexes and the mean number of ulcers per rat more effectively (p<0.001; 90.0 and 90.1 % inhibition, respectively) than D-002 (100 mg/kg).
Effects on neutrophil infiltration
Aspirin-positive control rats displayed mean values of 148.28 ± 7.9 (neutrophils per ulcerated area), which was dose-dependently lowered (R= 0.94) by D-002 treatment (table 2). All doses of D-002 (25, 50 and 100 mg/kg), reduced significantly the number of neutrophils per ulcerated area (41.7, 74.8 and 83.1 %, respectively, versus the aspirin positive control).
DISCUSSION
This study confirms that oral treatment with D-002 (25-100 mg/kg) protects against aspirin-induced gastric lesions and demonstrates, by first time, that D-002 reduces neutrophil infiltration on the gastric mucosa in rats with gastric injury induced by aspirin.
Gastric mucosal integrity is maintained by a dynamic process in which defensive factors should counteract the effects of aggressive factors. When this balance is lost, gastric mucosal damage becomes evident.1,2 Aspirin administration to rats, a classical model of NSAIDs-induced gastrotoxicity,14-17,31,32 mimics the potential gastric damage of the members of this therapeutic class. In particular, aspirin is the most widely used NSAIDs due its pivotal role in coronary and stroke prevention,33,34 in addition to the management of several inflammatory conditions.35
All positive controls exhibited the characteristic pattern of aspirin-induced gastric ulceration, such as erosions of the epithelium and inflammatory infiltrates around the ulcer area.31,32,36 Omeprazole 10 mg/kg, the reference treatment, reduced significantly the ulcer indexes, as reported by other authors.15,33 This supports the validity of this model in our conditions, and hence the present results, so that the effects here described herein can be attributable to D-002.
Oral treatment with D-002 dose-dependently prevented the development of aspirin-induced gastric ulcers, but only the 50 and 100 mg/kg doses were effective for lowering the gastric ulceration. The marked reduction (75 %) achieved with the highest dose (100 mg/kg), however, cannot be interpreted as a maximal inhibition because no plateau effect was seen and higher doses were not evaluated. Consistently, the mean number of ulcers per rat in the highest dose group was reduced by 71.9 %. These results are consistent with previous reports of the efficacy of D-002 for preventing NSAIDs-induced ulcers in rats.18,21
The effects of D-002 on aspirin-induced neutrophil infiltration in the gastric mucosa were dose-dependent and greater than its effects on the ulcer indexes, since all doses (25, 50 and 100 mg/kg) effectively reduced the number of neutrophils per ulcerated area (41.7, 74.8 and 83.1 %) as compared to the positive control. These results suggest that the gastroprotective effect of D-002 on this model is associated to the reduction of the neutrophil infiltration that accompanied the gastric ulceration induced by aspirin in rats.
It should be remembered that D-002 produces antioxidant effects on different rat tissues, including the reduction of lipid peroxidation in the gastric mucosa.21,37 Polimorphonuclear leukocytes represent one of the major sources of ROS in the gastric mucosa,12,16 then the present results should be attributable, at least partly, to the antioxidant effects of D-002. Nevertheless, D-002 has been shown to reduce LTB4 levels in pleural exudates of carrageenan-induced pleurisy,38 an effect that could be associated to the inhibition of the 5-LOX enzyme. Then, the reduction of LTs and derived ROS3,6-8 could be another explanation that could support the efficacy of D-002 on this model.
Oral treatment with D-002 (25-100 mg/kg) protects against aspirin-induced ulceration in rats, decreasing ulcer indexes and neutrophil infiltration in the gastric mucosa.
REFERENCES
1. Ramakrishnan K, Salinas RC. Peptic Ulcer Disease. Am Fam Physician. 2007;76:1005-12.
2. Tasman-Jones C. Pathogenesis of peptic ulcer disease and gastritis: Importance of aggressive and cytoprotective factors. Scand J Gastroenterol. 1986;19(22):1-5.
3. Lamarque D. Pathogenesis of gastroduodenal lesions induced by non-steroidal anti-inflammatory drugs. Gastroenterol Clin Biol. 2004;28:C18-26.
4. Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Cell Physiol. 2005;288:C1-19.
5. Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41-60.
6. Wallace JL. Pathogenesis of NSAID-induced gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol. 2001;15:691703.
7. Whittle BJ. Gastrointestinal effects of non-steroidal anti-inflammatory drugs. Fundam Clin Pharmacol. 2003;17:301-313.
8. Komatsu Y, Nakamori Y, Kotani T, Takeuchi K. Pathogenic importance of cysteinyl leukotrienes in development of gastric lesions induced by ischemia/reperfusion in mice. Gastroenterology. 2009:134;A-240.
9. Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48:743-51.
10. Demir S, Yilmaz M, Koseoglu M, Akalin N, Aslan D, Aydin A. Role of free radicals in peptic ulcer and gastritis. Turkish J Gastroenterology. 2003;14:39-43.
11. Yasukawa K, Kasazaki K, Hyodo F, Utsumi H. Non-invasive analysis of reactive oxygen species generated in rats with water immersion restraint-induced gastric lesions using in vivo electron spin resonance spectroscopy. Free Radic Res. 2004;38:147-55.
12. Naito Y, Takagi T, Katada K, Tomatsuri N, Mizushima K, Handa O, et al. Gastric peroxisome proliferator activator receptor-ã expression and cytoprotective actions of its ligands against ischemia-reperfusion injury in rats. J Clin Biochem Nutr. 2011;48:170-7.
13. Doweico HE. The Over-the-Counter Analgesics, Unexpected Agent of Abuse (Chapter eighteen) in Concept of chemical dependency. 7th ed. Belmont: Brooks/Cole Cengage Learning; 2009. p. 269.
14. Pohle T, Brzozowski T, Becker JC, Van Der Voort IR. Role of reactive oxygen metabolites in aspirin-induced gastric damage in humans: gastroprotection by vitamin C. Aliment Pharmacol Ther. 2001;15:677-87.
15. Sener-Muratoðlu G, Paskaloðlu K, Arbak S, Hürdað C, Ayanoðlu-Dülger G. Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig Dis Sci. 2001;46:318-30.
16. Jainu M, Devi S. Attenuation of neutrophil infiltration and proinflammatory cytokines by Cissus quadrangularis: a possible prevention against gastric ulcerogenesis. J Herb Pharmacother. 2005;5:33-42.
17. Jainu M, Mohan V. Devi S. Protective effect of Cissus quadrangularis on neutrophil mediated tissue injury induced by aspirin in rats. J Ethnopharmacol. 2006:104;302-5.
18. Carbajal D, Molina V, Valdés S, Arruzazabala L, Más R. Anti-ulcer activity of higher primary alcohols of beeswax. J Pharm Phamacol. 1995;47:731-3.
19. Carbajal D, Molina V, Valdés S, Arruzazabala L, Rodeiro I, Más R, et al. Possible cytoprotective mechanism in rats of D-002 an anti-ulcerogenic product isolated from beeswax. J Pharm Pharmacol. 1996;48:858-60.
20. Carbajal D, Molina V, Noa M, Valdes S, Arruzazabala ML, Aguiar A, et al. Effects of D-002 on gastric mucus composition in ethanol-induced ulcer. Pharmacol Res. 2000;42:329-32.
21. Molina V, Valdés S, Carbajal D, Arruzazabala L, Menéndez R, Más R. Antioxidant effects of D-002 on gastric mucosa of rats with injury induced experimentally. J Med Food. 2001;4(2):79-84.
22. Hano O, Illnait J, Mas R, Fernández L, Piñol F, Fernández JC. Effects of D-002, a Product Isolated from Beeswax, on Duodenal Ulcer: A Double-Blind, Placebo-Controlled Study. Curr Ther Res. 2001;62:394-407.
23. Illnait J, Terry H, Mas R, Fernández L, Carbajal D. Effects of D-002, a product isolated from beeswax, on gastric symptoms of patients with osteoarthritis treated with piroxicam: a pilot study. J Med Food. 2005;8:63-8.
24. Fernández L, Terry H, Quiñones AM, Díaz B, Hernández ML, Illnait J, et al. Effects of Abexol ® in middle-aged and older subjects: an open follow-up. Rev CENIC Cien Biol. 2008;39:3-8.
25. Rodriguez I, Illnait J, Fernandez L, Terry H, Mas R, Fernández L, et al. Effects of Abexol® (beeswax alcohols) on gastrointestinal symptoms of middle-aged and older subjects assessed. Rev CENIC Cien Biol. 2009;40(3):147-54.
26. Noa M, Más R, Carbajal D, Valdés S. Effect of D-002 on acetic acid-induced colitis in rats at single and repeated doses. Pharmacol Res. 2000;41:392-5.
27. Noa M, Carbajal D, Molina V, Valdés S, Más R. Comparative study of D-002 vs sulfasalazine on acetic acid-induced colitis in rats. Drugs Exptl Clin Res. 1999;25:25-9.
28. Angelo A, Hassan M, Nour El Din N, Khalifa HM, Ghany SA. A possible role for gastroprotectives on aspirin-induced gastric ulcer in rats. Alexandria J Med. 2010;46(1):75-82.
29. Ko JK, Leung CC.J. Ginger extract and polaprezinc exert gastroprotective actions by anti-oxidant and growth factor modulating effects in rats. Gastroenterol Hepatol. 2010;25:1861-8.
30. Nygard G, Amthony A, Piasecki C, Trevethick M, Hudson M, Dhilon A, et al. Acute indomethacin-induced jejunum injury in the rat. Early morphological and biochemical changes. Gastroenterology. 1994;106:567-75.
31. Mukherjee M, Bhaskaran N, Srinath R, Shivaprasad HN, Allan JJ, Shekhar D, et al. Anti-ulcer and antioxidant activity of GutGard. Indian J Exp Biol. 2010;48:269-74.
32. Ogawa K, Oyagi A, Tanaka J, Kobayashi S, Hara H. The Protective Effect and Action Mechanism of Vaccinium myrtillus L. on Gastric Ulcer in Mice. Phytother Res. 2011 Aug 25(8):1160-5.
33. Sanmuganathan P. Ghahramani P. Jackson P. Ramsay L. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-60.
34. Inzitari D, Piccardi B, Sarti C. A critical review of aspirin in the secondary prevention of non-cardioembolic ischaemic stroke. Int J Stroke. 2010;5:306-18.
35. Tsumura H, Tamura I, Tanaka H, Chinzei R, , Masuda A, et al. Prescription of non-steroidal anti-inflammatory drugs and co-prescribed drugs for mucosal protection: analysis of the present status based on questionnaires obtained from orthopaedists in Japan. Inter Med. 2007;46:927-31.
36. Berenguer B, Trabadela C, Sánchez-Fidalgo S, Quílez A, Miño P, De la Puerta R, et al. The aerial parts of Guazuma ulmifolia Lam. protect against NSAID-induced gastric lesions. Ethnopharmacol. 2007;114(2):153-60.
37. Menéndez R, Amor AM, González RM, Jiménez S, Más R. Inhibition of rat microsomal lipid peroxidation by the oral administration of D-002. Braz J Med Biol Res. 2000;33:85-90.
38. Carbajal D, Molina V, Valdés S, Arruzazabala ML, Más R, Magraner J. Anti-inflammatory activity of D-002: an active product isolated from beeswax. Prostagl Leukotr Essent Fatty Acids. 1998;59(4):235-8.
Recibido: 28 de noviembre de 2011.
Aprobado: 19 de enero de 2012.
Maikel Valle Clara. Centro de Productos Naturales, Centro Nacional de Investigaciones Científicas (CNIC). Calle 198 entre 19 y 21, Atabey, municipio Playa, La Habana, Cuba. Correo electrónico: maikel.valle@cnic.edu.cu