Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión impresa ISSN 0034-7515
Rev Cubana Farm vol.47 no.3 Ciudad de la Habana jul.-sep. 2013
PRODUCTO NATURAL
In vitro antiproliferative effect of fractions from the caribbean marine sponge Myrmekioderma gyroderma
Efecto antiproliferativo in vitro de fracciones obtenidas en un extracto de la esponja marina Myrmekioderma gyroderma existente en el mar Caribe
Dra. C. Diana Márquez Fernández,I Dra. C. Edna Márquez Fernández,II Dr. C. Olivier P. Thomas,III Dr. C. Alejandro Martínez MartínezI
I Facultad de Química Farmacéutica, Universidad de Antioquia. Medellín, Colombia.
II Universidad Nacional de Colombia Sede Medellín. Medellín, Colombia.
III Instituto de Química de Nice, Universidad de Nice-Sophia Antipolis. Nice, Francia.
ABSTRACT
Introduction: studies performed to Myrmekioderma genus sponges show phospholipid fatty acids, volatile compounds, sterols, bioactive cyclic diterpenes, sesquiterpenes, lineal diterpenes and glycolipid ethers.
Objetive: to evaluate the antiproliferative effect of seven fractions (F1-F7) obtained by flash column chromatography from the most bioactive extract of the sponge Myrmekioderma gyroderma, and to analyze the chemical composition of the most active fraction.
Methods: samples of dried sponge were extracted with two different solvents: CH2Cl2 (2 x 50 mL), and CH3OH (2 x 50 mL). Each fraction was evaluated on tumor cell derived cell lines; and the cell growth, and viability were determined by a colorimeter assay using sulforhodamine B. Fatty acids structure of the most active fraction was possible by GC-MS analysis of the methyl ester, and pyrrolidine derivatives.
Results: the fraction with higher activity on the assessed tumor cell lines is F4 due to it totally inhibited MDA-MB-231, and HT29 cell line growth to 5, and 25 µg/mL concentration (IC50< 1 µg/mL). Fatty acids identified in bioactive F4 fraction of the M. gyroderma sponge can be classified on the following groups: lineal chain saturated, branched-saturated, unsaturated, and a 3-hydroxy acid.
Conclusions: 43 fatty acids among saturated, branched-saturated, and unsaturated were identified out of the F4 fraction with activity on the cell lines derived of breast cancer MDA-MB-231, colon carcinoma HT29, and lung carcinoma cells A-549. These results show the growth inhibitory effect shown by the fractions, on the tumor cell lines, depends on the dose.
Key words: antiproliferative activity, fatty acids, Myrmekioderma gyroderma.
RESUMEN
Introducción: los estudios realizados a esponjas del género Myrmekioderma muestran la presencia de ácidos grasos de fosfolípidos, compuestos volátiles, esteroles, diterpenos cíclicos bioactivos, sesquiterpenos, diterpenos lineales y éteres glicolipídicos.
Objetivo: evaluar el efecto antiproliferativo de siete fracciones (F1-F7) obtenidas por cromatografía en columna instantánea del extracto más activo de la esponja Myrmekioderma gyroderma, y analizar la composición química de la fracción más activa.
Métodos: se extrajeron las muestras de esponja seca con dos solventes diferentes: CH2Cl2 (2 x 50 mL) y CH3OH (2 x 50 mL). Se evaluó cada fracción en líneas celulares derivadas de células tumorales, y se determinó el crecimiento celular y la viabilidad mediante un ensayo colorimétrico usando sulforhodamina B. Se realizó la identificación de los ácidos grasos de la fracción más activa mediante el análisis por cromatografía de gases acoplada a espectrometría de masas de los derivados ésteres metílicos y pirrolididas.
Resultados: la fracción más activa fue la F4, debido a que inhibió totalmente el crecimiento de las líneas celulares MDA-MB-231 y HT29 a 5 y 25 µg/mL (IC50< 1 µg/mL). Los ácidos grasos identificados se pueden clasificar en los siguientes grupos: saturados de cadena lineal, saturados-ramificados, insaturados y un hidroxiácido. Se identificaron 43 ácidos grasos entre saturados, saturados-ramificados e insaturados en la fracción con mayor actividad sobre las líneas celulares derivadas de cáncer de mama MDA-MB-231, carcinoma de colon HT29 y carcinoma de pulmón A-549.
Conclusiones: los resultados muestran que el efecto inhibitorio del crecimiento de las fracciones sobre las líneas celulares evaluadas depende de la dosis.
Palabras clave: actividad antiproliferativa, ácidos grasos, Myrmekioderma gyroderma.
INTRODUCTION
Myrmekioderma gyroderma sponge (class: Demospongiae, order: Halichondrida, family: Desmoxyidae) (Alcolado, 1984) lives in the Caribbean Sea.1 Studies performed on sponges Myrmekioderma genus show phospholipid fatty acids identification in M. dendyi,2 and M. styx3 sponges. Fatty acid, volatile compounds, and sterols present in the lipophilic extract of M. granulata sponge are active against several pathogenic microorganisms.4 Diterpenes, cyanthiwigins AA-AA, isolated from the M. styx sponge collected in Jamaica, showed activity against hepatitis B virus (HBV); Mycobacterium tuberculosis (Mtb) strain H37Rv at 43 mg/mL EC50, and 50 % inhibition at 6.25 mg/mL; the human immunodeficiency virus (HIV-1) with 42.1 mM EC50; and against human primary tumor cells at 3.1-18 mM IC50. Styxone A and B, styxlactone,5,6 lineal diterpenes,7 and glycolipid ethers8 were also isolated from the M. styx sponge.
Mono-O-alquyl-glycosyl glycerols, which cause reverting in tumor cell morphology;9 sesquiterpenes bisabolene type with inhibiting activity of the gastric ATPase,10 such as (S)-(+)-curcuphenol with activity on lines of cancer cells, enzymes, parasites, such as P. falciparum and microorganisms11 have been isolated from Myrmekioderma sp sponge. About Colombian sponges M. gyroderma and M. rea there is a study in which sterol compositions and their phylogenetic relationships were evaluated.1
Aiming to contribute with the search of antitumor compounds of potential interest in the cancer treatment, and to generate data on the sponge chemistry, the effect of fractions obtained from the ethanolic extract of Myrmekioderma gyroderma sponge on the growth of the breast (MDA-MB-231), colon (HT29), and lung (A549) tumor cell lines, was evaluated; and the chemical composition of the most active fraction was analyzed.
METHODS
General experimental procedures
Solvents employed for extraction and fractions were analysis grade (Merck®), and HPLC-grade solvents were used without further purification in HPLC separations. HPLC purifications were carried out on a Waters equipment (pump 600 E, autoinjector 417, and photodiode array detector 996) coupled with an evaporative light-scattering detector (ELSD) SEDEX 55. The following chromatographic conditions were used: a Phenomenex® column C18 (250 mm x 10 mm, 5 µm), using as mobile phase acetonitrile/water (30:70) under isocratic conditions for 5 minutes, later gradient mode for 10 min in acetonitrile/water (80:20), and later under isocratic conditions for 25 min in 100 % acetonitrile. A 3 mL/min flow rate; 100 µL volume injection; and 210 nm wavelength detector were used. NMR experiments were performed on a Bruker ARX 500 spectrometer. Chemical shifts are recorded in ppm with CDCl3 (d 7.26 for 1H) as internal standard.
The analyses by gas chromatography - mass spectrometry (GC-MS) were performed in an Agilent® 6890N gas chromatographer coupled to an Agilent® 5973N mass spectrometer. For the (GC-MS) analysis of the methyl esters of the fatty acids, a 19091S, HP5MS Agilent® (0.25 mm x 30 m x 0.25 µm) column at a maximum 350 ºC temperature was used. The schedule of the oven was as follows: starting temperature 150 ºC at a 10 ºC gradient up to a final 300 ºC temperature. Splitless mode at 200 ºC temperature, 13.2 psi pressure, and 14.1 mL/min total flow for the injection. The auxiliary detector temperature was 300 ºC. The scan mode in the mass detector was used at a mass interval of 30-600 amu. The injection volume was 5.0 µL, and the analysis time was 35 minutes. For the GC-MS analysis of the pyrrolidine derivatives from the fatty acids, a 19091J-433, HP-5 Agilent® (0.25 mm x 30 m x 0.25 µm) column was used at 350 ºC top temperature. The schedule of the column was as follows: starting temperature 150 ºC (1 min)-200 ºC (5 ºC/min-3 min)-250 ºC (3 ºC/min-4 min)-300 ºC (5 ºC/min-5.33 min). Splitless mode was used for the injection. The injector's temperature was 250 ºC, 13.1 psi pressure, and 14.1 mL/min total flow. Auxiliary detector's temperature was 300 ºC. Scan mode in the mass detector at 30-600 amu mass interval was used. The injection volume was 3.0 µL, and the analysis time was 90 minutes.
Sampling and identification
Samples were collected by scuba divers from reef in habitats at 15 to 21 m depths of in the Urabá Gulf (Colombia), in 2002 and were kept frozen until their use. Strange materials and/or organisms were removed from samples with a knife. Samples were frozen (-10 °C) as soon as possible and transferred to the laboratory where they were cut in small pieces, and dried (40 °C). Identification was carried out by Dr. Sven Zea (Colombia), sponge taxonomy expert, and two reference samples are in the Laboratorio de Productos Naturales Marinos de la Universidad de Antioquia (Medellín, Colombia) with voucher Nº PNM-23.
Extracting and fractioning
Dried samples were extracted with two different solvents: CH2Cl2 (2 x 50 mL), and CH3OH (2 x 50 mL). Each extraction was developed with mechanical shaking in amber glass flasks at room temperature. Each extract was filtered, and concentrated under vacuum on a rotary evaporator (Heidolph) at low temperature (40 °C). The ethanol extract was subjected to a C18 reversed-phase flash column, eluting with 500 mL of the following eluotropic series: H2O, H2O/CH3OH (1:1), H2O/CH3OH (1:3), CH3OH, CH3OH/CH2Cl2 (3:1), CH3O H/CH2Cl2 (1:1), and CH2Cl2. Each fraction was concentrated under vacuum on a rotary evaporator at low temperature (40 °C), and later evaluated on tumor cell derived cell lines. Fractions were named in the same order each solvent elution was, that is: F1, F2, F3, F4, F5, F6, F7; and the F4 fraction was separated by reversed-phase HPLC.
Biological assay
The cell growth and viability were determined by a colorimeter assay using sulforhodamine B, SRB.12 The colorimetric assay estimates cell number indirectly by staining total cellular protein with the dye SRB (12). The in vitro activity of the extracts was evaluated against cultured human cancer cells of A-549 lung carcinoma, HT29 colon adenocarcinoma, and MDA-MB-231 breast carcinoma at three concentrations: 1 µg/mL, 5 µg/mL, and 25 µg/mL. According to National Cancer Institute guidelines, extracts and fractions with IC50 values < 20 µg/mL were considered active.13
Preparation of methyl esters and pyrrolidine derivatives of the fatty acids
The HPLC obtained fractions of fatty acids from the M. gyroderma sponge were added diazomethane aiming to get methyl esters from the fatty acids. 100 µg of methyl esters were added 1 000 µL of pyrrolidine, and 100 µL glacial acetic acid for getting pyrrolidine derivatives. The mix was boiled at 100 ºC in an open reflux for 90 minutes. Reaction was monitored using thin layer chromatography comparing with a sample of methyl esters using hexane/ethyl acetate (2:1) as mobile phase, and silica gel F254 as stationary phase. Both derivative samples were analyzed by GC-MS.
RESULTS
Bioassays
Figure 1 shows M. gyrodermasponge fraction with the highest activity on the assessed tumor cell lines is F4 since it totally inhibited MDA-MB-231, and HT29 cell line growth to 5, and 25 µg/mL concentration(IC50< 1 µg/mL), respectively.
Fractionation of F4 fraction by HPLC
The HPLC fractioning of the most active fraction (F4) resulted on the collection of sixteen fractions named M1 to M16. M. gyroderma sponge HPLC obtained fractions were analyzed by 1H-NMR, and fatty acid characteristic signals were observed on the obtained spectra. Fatty acids structure identification was possible by GC-MS analysis of the methyl ester, and pyrrolidine derivatives. 43 fatty acids (C14-C21) distributed in the different fractions obtained by HPLC were identified from the fraction of free fatty acids of the M. gyroderma sponge. Results are shown in table.
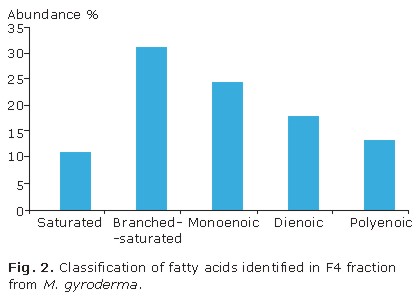
Fatty acids identified in bioactive F4 fraction of the M. gyroderma sponge can be classified on the following groups: lineal chain saturated, branched-saturated, unsaturated, and a 3-hydroxy acid. Figure 2 shows fatty acids that are more abundant are in order: branched-saturated (32.5 %), monoenoic (25.6 %), dienoic (18.6 %), polyenoic (14.0 %), and lineal chain saturated (11.6 %). Unsaturated fatty acids are the most commonly found in the fraction and correspond to 55.8 % abundance.
DISCUSSION
A549 cell line was totally inhibited on its growth at a 25 µg/mL concentration, and at 5 µg/mL one, the same fraction inhibited its cell line growth in 71 % (IC50 3.10 µg/mL). 1 µg/mL concentration completely inhibited HT29 cell line growth, and diminished 72 % MDA-MB-231, and 22 % A549 cell line growth; respectively. For its part, F3 fraction inhibited 76 %, 71 % and 12 % MDA-MB-231, HT29 and A549 cell line growth; respectively. On the other hand F5 fraction inhibited 65 %, 39 %, and 45 % MDA-MB-231, HT29 and A549 cell line growth, respectively, at a 25 µg/mL concentration. F6 fraction inhibited 26 % A549 cell line growth, and the other cell lines were inhibited in less proportion.
Fatty acid characteristic signals observed on the obtained spectra were: a triplet d 0.8-0.9 ppm signal, corresponding to methyl group; a d 1.2-1.4 ppm characteristic signal, of methylene groups; the displaced multiplet signals between d 1.5 ppm, and d 3.0 ppm, corresponding to methyl groups close to carboxyl group; being a triplet signal evident in d 2.3 ppm, corresponding to the two protons of the methyl group directly linked to the carbonyl group.14 The proton of the hydroxy-bearing carbon generated a multiplet at d 3.50 ppm.15 Olefinic protons characteristic signals were also observed due to the presence of a multiplet removed to d 5.3 ppm.16
The mass spectra of methyl ester derivatives allowed determining whether the compound was a saturated fatty acid by observing the presence of characteristic fragments such as: m/z 74 base peak, which comes from a McLafferty type rearrangement; the ion at m/z [M-43]+, which represents the loss of C3 units (2 to 4 carbons); and the ion at m/z [M-31]+ , which represents the loss of a methoxyl ion.17 The mass spectra of methyl esters of unsaturated fatty acids showed the presence of an ion at m/z [M-32]+, which represents the loss of methanol; the ion at m/z 74, which is less abundant than in saturated fatty acids; and the ion at m/z 55, which is usually the base peak of the [CnH2n-1]+ series.14 The pyrrolidine derivatives allowed the location of the double bonds, and branches.16 A double bond is present between the n and n+1 carbons in the molecule, if a 12 amu rather than a 14 amu interval is observed, among the most intense peaks of the fragments containing n and n-1 carbon atoms.16
Mass spectrum analysis of the methyl esters of the free fatty acid obtained from the M. gyroderma sponge revealed the presence of fatty acids, which showed a base peak of m/z 81, a diagnostic ion of fatty acids with D5,9 unsaturations, which is a characteristic pattern of the demospongic acids.18 This pattern was observed in the mass spectrum of the pyrrolidine derivatives of the same fatty acids in which the m/z 180 ion was observed, which is characteristic of the D5,9 fatty acids.18 The identification of the compounds was based on the comparison of the published mass spectra, and retention times.14
For the case of the hydroxylated fatty acid identified in the M1 fraction, methyl ester mass spectrum showed the m/z 286 molecular ion, corresponding to the molecular formula C17H34O2. The base peak at m/z 103 is produced by a characteristic cleavage alpha to the carbon with the hydroxyl group, and defines its position.17
Composition of fatty acids identified in this work was compared with composition reported for the phospholipid fraction of the M. styx sponge,3 and it was found that both fraction common fatty acids are: 8-Me-C15:0, C16:2; D5,9, C18:0, C20:4; n-6, C16:0, ai-C15:0, i-C15:0, ai-C16:0, i-C17:0, ai-C17:0, C17:0. A 25.6 % similarity between both fractions was observed.
Biological activity results indicate that the greatest inhibitory effect in growth was observed in HT29 (IC50< 1.00 µg/mL) tumor cells, which growth was totally inhibited by F4 fraction in the three assessed concentrations (1, 5 and 25 µg/mL); it was followed by MDA-MB-231 (IC50< 1.00 µg/mL) tumor line with total growth inhibition at 5 and 25 µg/mL concentrations; and finally A549 (IC50 3.10 µg/mL) cell line, which growth was totally inhibited at a 25 µg/mL concentration. The most sensible cell line was MDA-MB-231, as it is the only cell line showing more than 50 % inhibition for three out of four assessed fractions (F4, F3, and F5), cell line HT29 (F4 and F3) follows, and finally A549 (F4) cell line.
Comparison of the obtained IC50 with the National Cancer Institute extract suggested values of 20 µg/mL;13 it was found that F4 fraction exhibited antiproliferative activity in the three assessed cell lines, which lead to chemical study for determining its composition.
Three groups of fatty acids showing greater abundance, and which could be responsible of the analyzed tumor lines growth inhibition, were found in F4 fraction: hexadecanoic or palmitic acid (37.16 %); monounsaturated fatty acids (C14-C22), among which the most abundant are the hexadecenoic (19.38 %), and 10-octadecenoic (14.79 %) acids; and iso and anteiso branched-saturated fatty acids C15-C17.
The most abundant component of the analyzed fraction is the hexadecanoic (palmitic acid) which, at a 12.5 to 50 µM/mL concentration, showed selective cytotoxic activity against human leukemia cells (MOLT-4, HL60, K-562), but not on normal cells HDF; it also induces apoptosis in MOLT-4 cell lines at 50 µg/mL concentration, and presents in vivo antitumor activity in mice trials. It is considered that a molecular target for this compound is the topoisomerase I, and that it does not affect topoisomerase II.19
Monounsaturated fatty acids were found in abundance in F4 fraction (36.34 %), and it is possible that these compounds have cis position double link, which would partially explains the activity observed in such fraction, due to what has been reported that cis-monounsaturated fatty acids (C14-C22) are known as DNA topoisomerase I inhibitors.20 Besides, it has been reported that cis-C18:1 fatty acid is an inhibitor of the human telomerase with an IC50 of 8.6 µM;21 and the presence of 8-octadecenoic, and 10-octadecenoic, with < 0.10 %, and 14.79 % abundance respectively, was found in F4 fraction. 9,12-octadecadienoic acid was also found in the same fraction, and it has also been reported that this acid with the first double link in cis configuration becomes a mammal DNA polymerase inhibitor.22
C15-C17 (iso and anteiso) branched-saturated fatty acids identified in bioactive F4 fraction also contribute to antiproliferative activity, as it has been reported that these compounds have inhibitory activity on topoisomerase I.23 15-methylhexadecanoic acid (i-C17:0) with 8.44 % abundance, 14-methylhexadecanoic acid (ai-C17:0) with 0.79 % abundance; and 13-methyltetradecanoic acid (i-C15:0), 12-methyltetradecanoic acid (ai-C15:0), 14-methylpentadecanoic acid (i-C16:0), and 13-methylpentadecanoic acid (ai-C16:0) with an abundance less than 0.10 % each, are found in the tested fraction.
Another antiproliferative interest fraction component is 5,8,11,14,17-eicosapentaenoic acid (n-3 acid), which is found in traces in M2 fraction (< 0.10 %). It has been indicated that n-3 fatty acids are powerful inhibitors of lipoxygenase-enzymes involved in illnesses such as Alzheimer, arteriosclerosis, and cancer.24
3-hydroxyhexadecanoic acid presence suggests the existence of an endosymbiotic relationship between the sponge, and a microorganism; as this kind of fatty acids (3-hidroxylated) has been found in microbial lipids (rhamnolipids).14,25 3-hidroxylated fatty acids have shown antimycotic activity between 10 and 100 µg/mL MIC.25
In conclusion, 43 fatty acids (among saturated, branched-saturated, and unsaturated), were identified out of the F4 fraction with activity on the cell lines derived from breast cancer MDA-MB-231, colon carcinoma HT29, and lung carcinoma cells A-549. This is the first report on biological activity of fractions of the Myrmekioderma gyroderma sponge on cell lines derived from breast cancer MDA-MB-231, colon carcinoma HT29, and lung carcinoma cells A-549; and on the chemical composition of a bioactive fraction of this sponge. These results illustrate that the growth inhibitory effect shown by the fractions on the tumor cell lines depends on the dose.
Acknowledgments
Authors thank to professor Sven Zea, of the Universidad Nacional de Colombia, for the animal material identification; and the Universidad de Antioquia, the Universidad Nacional de Colombia sede Medellín, and Colciencias for the financing of the project 11150520268. This work fulfills what is stated in the contract of access to derived product for scientific research with no commercial interest N° 28 between the Ministerio de Ambiente, Vivienda y Desarrollo Territorial; and Alejandro Martínez M., professor at the Universidad de Antioquia.
REFERENCES
1. Castellanos L, Zea S, Osorno O, Duque C. Phylogenetic analysis of the order Halichondria (Porifera, Demospongiae), using 3b-hydroxysterols as chemical characters. Biochem Systemat Ecol. 2003;31:1163-83.
2. Genin E, Wielgosz-Collin G, Njinkoué JM, Velosaotsy NE, Kornprobst JM, Gouygou JP, et al. New trends in phospholipid class composition of marine sponges. Comp Biochem Physiol. 2008;B 150:427-31.
3. Carballeira NM, Reyes ED, Shalabi F. Identification of novel iso/anteiso nonacosadienoic acids from the phospholipids of the sponges Chondrosia remiformis and Myrmekioderma styx. J Nat Prod. 1993;56:1850-5.
4. Mishra PM, Sree A, Baliarsingh S. Antibacterial study and fatty acid analysis of lipids of the sponge Myrmekioderma granulata. Chem Nat Comp. 2009;45:621-4.
5. Peng J, Franzblau SG, Zhang F, Hamann MT. Novel sesquiterpenes and a lactone from the Jamaican sponge Myrmekioderma styx. Tetrahedron Lett. 2002;43:9699-702.
6. Peng J, Avery MA, Hamann MT. Cyanthiwigin AC and AD, two novel diterpene skeletons from the Jamaican sponge Myrmekioderma styx. Organic Lett. 2003;5:4575-8.
7. Albrizio S, Fattorusso E, Magno S, Mangoni A. Linear diterpenes from the Caribbean sponge, Myrmekioderma styx. J Nat Prod. 1992;55:1287-93.
8. Letourneux Y, Brunel JM, Fernández R, Dherbomez M, Debitus C. Isolation and characterization of new tetrahydropyranyl substituted sesquiterpene and Myrmekiodermin glycolipid ether isolated from the marine sponge Myrmekioderma. Heterocycl Comm. 2005;11: 291-8.
9. Aoki S, Higuchi K, Kato A, Murakami N, Kobayashi M. Myrmekiosides A and B, novel mono-O-alkyl-diglycosylglycerols reversing tumor cell morphology of ras-transformed cells from a marine sponge of Myrmekioderma sp. Tetrahedron. 1999;55:14865-70.
10. Fusetani N, Sugano M, Matsunaga S, Hashimoto K. (+)-Curcuphenol and dehydrocurcuphenol, novel sesquiterpenes which inhibit H,K-ATPase, from a marine sponge Epipolasis sp. Experientia. 1987;43:1234-5.
11. Gul W, Hammond NL, Yousaf M, Peng J, Holley A, Hamann MT. Chemical transformation and biological studies of marine sesquiterpene (S)-(+)-curcuphenol and its analogs. Biochim Biophys Acta. 2007;1770:1513-9.
12. Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113-8.
13. Boyd MR. The NCI in vitro anticancer drug discovery screen: concept, implementation, and operation, 1985-1995. In: Teicher BA, editor. Anticancer drug development guide; preclinical screening, clinical trials and approval. Totowa: Humana Press; 1997. p. 23-42.
14. Christie WW. The Lipid Library. [Internet]. The American Oil Chemists Society. 2010. Available from: http://www.lipidlibrary.co.uk
15. Jakob B, Voss G, Gerlach H. Synthesis of (S)- and (R)-3- hydroxyhexadecanoic acid. Tetrahedron Asymmetry. 1996;7:3255-62.
16. Andersson BA, Holman RT. Pyrrolidides for mass spectrometric determination of the position of the double bond in monounsaturated fatty acids. Lipids. 1974;9:185-90.
17. Ryhage R, Stenhagen E. Mass spectrometric studies. VI. Methyl esters of normal chain oxo-, hydroxy-, methoxy- and epoxy-acids. Arkiv Kemi. 1960;15:545-74.
18. Carballeira NM, Negrón V, Reyes ED. Novel monounsaturated fatty acids from the sponges Amphimedon compressa and Mycale laevis. J Nat Prod. 1992;55:333-9.
19. Harada H, Yamashita U, Kurihara H, Fukushi E, Kawabata J, Kamei Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22:2587-90.
20. Suzuki H, Yosida TH. Frequency of sister-chromatid exchanges depending on the amount of 5-bromodeoxyuridine incorporated into parental DNA. Mutation Res. 1983;111:277-82.
21. Oda M, Ueno T, Kasai N, Takahashi H, Yoshida H, Sugawara F, Sakaguchi K, Hayashi H, Mizushina Y. Inhibition of telomerase by linear-chain fatty acids: a structural analysis. Biochem J. 2002;367:329-34.
22. Mizushina Y, Tanaka N, Yagi H, Kurosawa T, Onoue M, Seto H, et al. Fatty acids selectively inhibit eukaryotic DNA polymerase activities in vitro. Biochim Biophys Acta. 1996;1308:256-62.
23. Lee HK, Lee DS, Kim J, Kim JS, Im KS, Jung JH. Topoisomerase I inhibitors from the Streptomyces sp. strain KM86-9B isolated from a marine sponge. Arch Pharm Res. 1998:21:729-33.
24. Jacquot C, McGinley CM, Plata E, Holman TR, van der Donk W. Synthesis of 11-thialinoleic acid and 14-thialinoleic acid, inhibitors of soybean and human lipoxygenases. Org Biomol Chem. 2008;6:4242-52.
25. Yano I, Ohno Y, Masui M, Kato K, Yabuuchi E, Ohyama A. Occurrence of 2- and 3-hydroxy fatty acids in high concentrations in the extractable and bound lipids of Flavobacterium meningosepticum and Flavobacterium IIb. Lipids. 1976;11:685-8.
Recibido: 23 de marzo de 2013.
Aprobado: 29 de mayo de 2013.
Diana Márquez. Facultad de Química Farmacéutica, Universidad de Antioquia. Calle 57 No. 53-108, Bloque 2, Laboratorio 131. Apartado Aéreo 1226. Medellín, Colombia.