My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Cubana de Farmacia
Print version ISSN 0034-7515
Rev Cubana Farm vol.47 no.4 Ciudad de la Habana Dec. 2013
PRODUCTO NATURAL
Nitric oxide inhibitory activity of hydrogenated synthetic analogues of furanonaphthoquinones isolated from Tabebuia spp.
Efecto inhibidor de los análogos sintéticos hidrogenados de las furanoaftoquinonas aisladas del género Tabebuia spp. sobre la producción de óxido nítrico
Ph.D. Luis Alberto Franco Ospina, Ph.D. Yanet Cecilia Ocampo Buendía, Ph.D. Ricardo Gaitán Ibarra
Faculty of Pharmaceutical Sciences, University of Cartagena. Cartagena, Colombia.
ABSTRACT
Objective: to describe the synthesis of analogues of furanonaphthoquinones isolated from Tabebuia genus and their inhibitory effect on nitric oxide production.
Methods: a series of six derivatives were prepared through cycloaddition reactions and the products characterized by spectroscopy methods. The biological activity was evaluated measuring their effect on the pro-inflammatory mediator production in macrophages RAW 264.7 induced with lipopolysaccharides. To prevent compounds from interfering with cellular viability, their cytotoxic effect was determined using methyl tetrazolium assay. Additionally, scavenging effect was in vitro measured.
Results: FNQ1, FNQ2, and FNQ5 derivatives showed potent concentration-depending inhibitory effect on nitric oxide production, with an IC50 value lower than 2 µM concentration at which they did not have toxic or scavenging effects. FNQ5 was the most active and selective derivative.
Conclusions: this is the first paper concerning the anti-inflammatory potential of tested synthetic compounds. Our results indicated that FNQ5 might be considered as useful potential anti-inflammatory molecule to treat inflammatory diseases related with nitric oxide overproduction.
Key words: furanonaphthoquinones, synthesis, Tabebuia, antiinflammatory agents, nitric oxide (NO•).
RESUMEN
Objetivo: describir la síntesis de análogos de furanonaftoquinonas aisladas del género Tabebuia y su efecto inhibidor en la producción de óxido nítrico.
Métodos: se obtuvo una serie de seis derivados a través de reacciones de cicloadición y se caracterizaron los productos por métodos espectroscópicos. Se evaluó la actividad biológica por su efecto en la producción del mediador proinflamatorio en macrófagos RAW 264.7 activados con lipopolisacárido. Para asegurar que los compuestos no interfirieran con la viabilidad celular, se evaluó su efecto citotóxico empleando el ensayo de metiltetrazolio. Adicionalmente, se evaluó el efecto captador del radical in vitro.
Resultados: los derivados FNQ1, FNQ2 y FNQ5 demostraron potente efecto inhibitorio en la producción de óxido nítrico de manera concentración-dependiente, con un valor de CI50 menor que 2 µM, concentración a la que no ejercieron efectos tóxicos o captadores de radicales. FNQ5 resultó el compuesto más activo y selectivo.
Conclusiones: este trabajo es el primero que evalúa el potencial antinflamatorio de los compuestos sintetizados. Los resultados indican que FNQ5 puede ser considerada como una molécula de uso potencial para el tratamiento de enfermedades inflamatorias que cursen con sobreproducción de óxido nítrico.
Palabras clave: uranonaftoquinonas, síntesis, Tabebuia, agentes antinflamatorios, óxido nítrico (NO•).
INTRODUCTION
Inflammation is a complex and delicate mechanism composed of cellular immunity and biochemical mediators with interrelated biological effects that occur as a response to injury, infection, and stress.1,2 However, excessive production of inflammatory mediators during chronic inflammation contributes to the pathogenesis and development of some diseases such as cardiovascular and bowel diseases, cancer, diabetes, arthritis, and neurodegenerative disorders that affect a significant part of the human population.3,4 Steroids and non-steroidal anti-inflammatory drugs (NSAID) are the most clinically important drugs to treat inflammatory diseases, which are associated with a high incidence of adverse effects.5 This justifies research directed at study and identification of new active substances safer and more effective to prevent and treat inflammatory disorders and related conditions.
Tabebuia spp. (Bignoniaceae) includes approximately 100 species, known as strictly woody, found in tropical rain forest areas throughout Central and South America.6,7 The products obtained from the inner bark of several species of this genus are popularly called Taheebo, Lapacho, Pau d'Arco, and Ipe roxo, and have been traditionally used as a poultice or concentrated tea for treating a variety of diseases associated with an inflammatory component.8,9 This traditional use has been validated by studies which identified several fractions obtained from these plants with anti-inflammatory, astringent, anti-bacterial and anti-fungal effect.8,10,11 In our previous report, we demonstrated the anti-inflammatory activity of ethanol extracts and some fractions from Tabebuia rosea and Tabebuia ochracea using the 12-o-tetradecanoylphorbol-13-acetate induced ear edema in mice.12 The major active compounds identified in the bark extracts from Tabebuia spp. include naphthoquinones, furanonaphthoquinones (FNQ), anthraquinones, benzoic acid and benzaldehyde derivatives, iridoids, coumarins, and flavonoids.11,13 Among these compounds, FNQ derivatives are an important group of molecules, widely distributed in nature and associated with a broad range of biological activities.14 The experimental evidence has demonstrated the anti-inflammatory potential of FNQ by inhibition of cytokine release,15,16 mast cell degranulation,17 and nitric oxide (NO•) and prostaglandin E2 (PGE2) production by suppression of NF- êB.18
In previous work, our research group reported the isolation and identification of a series of FNQ with antimalarial and immunomodulatory activity from the stem inner bark of several species of Tabebuia genus.19-21 However, the isolation yield of compounds from Tabebuia extracts was very low and limited their use in assays of biological activity. This situation encourages us to continue our studies obtaining five synthetic analogues of FNQ isolated from Tabebuia genus and the evaluation of their potential NO• inhibitory activity. Structural variations were introduced in order to reflect changes in the biological activity of dihydrofuranonaphthoquinone derivatives with different type and number of substitutions at the 2 and 3 position of the dihydrofuran ring.
METHODS
2-hydroxy-1,4-naphthoquinone (lawsone), cerium (IV) ammonium nitrate (CAN), silica gel for column chromatography (Merck grade 9385, 230-400 mesh), Dulbecco´s Modified Eagle Medium (DMEM), L-glutamine, antibiotics (Penicillin-Streptomycin), dimethyl sulfoxide (DMSO), trypan blue, lipopolysaccharides from Escherichia coli serotype 0127:B8 (LPS), (N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide dihydrochloride (1400W), sodium nitrite (NaNO2), sodium nitroprusside (SNP), N-(1,1-naphthyl)ethylenediamine dihydrochloride, sulfanilamide, and Phosphate Buffer Saline tablets were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from GIBCO (Gaithersburg, MD, USA), and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tertazolium bromide (MTT) from Calbiochem®(USA). Organic solvents were analytical grade and obtained from Mallinckrodt Baker (San Diego, CA, USA).
SYNTHESIS OF FNQ COMPOUNDS
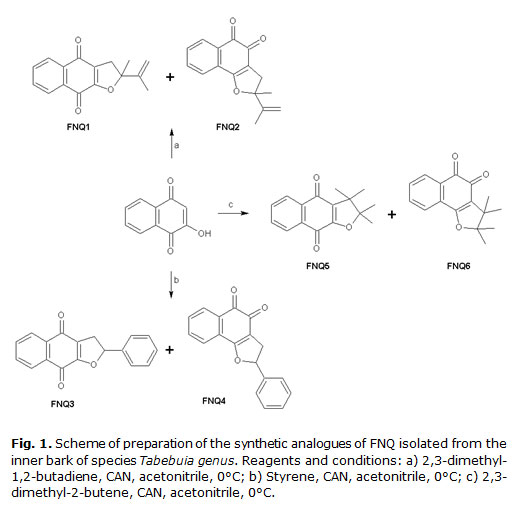
The analogues of FNQ isolated from Tabebuia genus, were synthesized according to the methods previously reported.22-24 The synthetic route used to prepare the different molecules employed 2-hydroxy-1,4-naphthoquinone (lawsone), as starting material, and was based on cycloaddition reactions [3+2] in the presence of cerium (IV) ammonium nitrate (CAN), alkenes, alkynes and acetonitrile as solvent (Figure 1). All reactions were conducted under inert atmosphere conditions and products purified by column chromatography on silica gel.
Compounds were unambiguously characterized by spectroscopy methods and uncorrected melting points (m.p.) determined by Differential Scanning Calorimetry-DSC7 (Perkin-Elmer, USA), assigning the corresponding structures and their physicochemical characteristics that in all cases coincided with those previously reported: 2-methyl-2-vinyl-2,3-dihydronaphtho[2,3-b]furan-4,9-dione (FNQ1), bright orange needles (27.3 %), m.p.: 149 °C; 2-methyl-2-vinyl-2,3-dihydronaphtho[1,2-b]furan-4,5-dione (FNQ2), yellow needles (35.8 %), m.p.; 2-phenylnaphtho[2,3-b]furan-4,9-dione (FNQ3), yellow crystals (47 %), m.p.: 158 °C; 2-phenyl-2,3-dihydronaphtho[1,2-b]furan-4,5-dione (FNQ4), dark orange crystals (27 %), m.p.: 115 °C; 2,2,3,3-tetramethyl-2,3-dihydronaphtho[2,3-b ]furan-4,9-dione (FNQ5), yellow crystals (48.9 %) m.p.: 91 °C; 2,2,3,3-tetramethyl-2,3-dihydronaphtho[1,2-b ]furan-4,5-dione (FNQ6), orange crystals (12.5 %) m.p.: 101 °C. FNQ5 and FNQ6 compounds could not be resolved into their corresponding stereoisomers.
All of the test compounds were dissolved in dimethylsulfoxide (DMSO) to obtain a stock solution, and stored as small aliquots at -20 °C. The compounds were diluted serially to the appropriate final concentration with supplemented culture medium, just before cell exposure with final concentration ranging from 10 µM to 0.01 µM, except for FNQ3 which was tested at a maximum concentration of 1 µM because its low solubility did not permit preparation of an appropriate stock solution. The final percentage of DMSO was adjusted to 0.1 % (v/v). FNQ6 was not tested because of its low yield.
DETERMINATION OF THE PARTITION COEFFICIENT (logP)
Lipid solubility was theoretically calculated for each of the molecules evaluated by their logP values, using a free online server available on the website http://www.vcclab.org/lab/alogps/.
CELL CULTURE
The murine RAW 264.7 macrophage-like cell line was obtained from the American Type Culture Collection (TIB-71; Rockville, MD, USA) and routinely cultured in DMEM supplemented with 2 mM L-glutamine, antibiotics (100 IU/mL of penicillin-100 µg/mL streptomycin) and heat-inactivated fetal bovine serum (FBS) at 37 °C in a humidified atmosphere containing 5 % CO2 and 95 % air.
MTT REDUCTION ASSAY
Cytotoxic effect of test compounds was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test (MTT assay).25 RAW 264.7 macrophages (2 x 105 cells/mL) were plated into 96-well plates and allowed to grow at 37 °C in 5 % CO2 atmosphere. Subsequently, the culture medium was replaced with various concentrations of test compounds for 30 min, followed by stimulation with of E. coli lipopolysaccharide (LPS; 10 µg/mL) and incubated for 24 h. Triton X-100TM (2 %) was used as positive control. After incubation, treatment was retired and replaced by medium containing MTT (0.25 mg/mL). Four hours later, the medium was carefully aspirated and formazan crystals were dissolved in DMSO. Optical Density at 550 nm (OD550) was measured using a microplate reader (Multiscan EX Thermo®). Percentages of cell survival relative to control group were calculated, as well as the concentration that reduces survival to 50 % (LC50).
NO• PRODUCTION
The evaluation of the NO• inhibitory activity was performed in a manner similar to that described for cell viability. In brief, RAW 264.7 cells were seeded in 24-well plates (2 x 105 cells/mL) and allowed to grow at 37 °C in 5 % CO2 atmosphere. The adherent cells were treated for 30 min with various concentrations of test compounds or (N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide dihydrochloride (1 400W), as positive control, and stimulated with LPS (10 µg/mL). Twenty four hours later, culture supernatants were collected and stored at -20 °C until use.
Nitrite (NO2-) accumulation, as an indicator of NO• production in the medium, was determined by the Griess method.26 Briefly, 100 µL of supernatants were mixed with an equal volume of Griess reagent (1:1 mixture of 0.1 % N-(1-naphthyl) ethylenediamine dihydrochloride and 1 % sulfanilamide in 5 % H3PO4) and incubated at room temperature for 5 min. The OD550 of the samples was measured using a microplate reader (Multiscan EX Thermo®). The amount of nitrite in the samples was calculated from a standard curve of sodium nitrite (NaNO2). Percentage of inhibition was calculated against cells that were not treated but were induced with LPS. The concentration that inhibited the LPS-stimulated NO• production by 50 % (IC50) was determined.
SCAVENGING OF NO•
This assay was carried out in order to evaluate the effect of synthetic FNQ on NO• in a cell-free system. Sodium nitroprusside (SNP) was used to generate NO• in an aqueous solution at physiological pH, which was detected by Griess reagent after reaction with dissolved oxygen to form NO2-. Scavengers of NO• compete with oxygen, leading to reduce production of NO2-.27 Test compounds were incubated with 1 mL of SNP (5 mM) in PBS at 25 ºC for 120 min. Samples (100 µL) were incubated at room temperature for 5 minutes with an equal volume of Griess reagent. The OD550 of the samples was measured using a microplate reader (Multiscan EX Thermo®) and compared with standard solutions of NaNO2 to calculate the nitrite concentration.
STATISTICAL ANALYSIS
All values are expressed as mean ± standard error of the mean (S.E.M.). LC50 and IC50 values were calculated using non-linear regression analysis and expressed as mean and its 95 % confidence interval. Selectivity index (SI) was calculated as the ratio LC50/IC50. Data were analyzed using one-way analysis of variance (ANOVA), followed by Dunnett´s post hoc test, to determine the differences between groups. Values of p< 0.05 were considered significant.
RESULTS
SYNTHESIS OF FNQ COMPOUNDS
Six analogues of FNQ isolated from Tabebuia genus were prepared as shown in Figure 1, obtaining a mixture of linear and angular conformational isomers, with yields varying between 12.5 % and 48.9 %. The lowest yields were obtained with compounds FNQ4 and FNQ6, 27 % and 12.5 %, respectively, which indicates the dominance of linear isomer. As previously reported, the high regioselectivity of cyclization can be easily explained by the mechanism of the reaction: initial formation of radicals followed by oxidation an formation of carbocations intermediates which react intramolecularly with the hydroxyl groups to produce linear and angular isomers, ortho- and para-quinones (Figure 2). All compounds were characterized and their physicochemical properties coincided with those previously reported in the literature.22,23
EFFECT OF SYNTHETIC ANALOGUES OF FNQ ISOLATED FROM TABEBUIA ON CELL VIABILITY
DMSO, used as vehicle, did not produce any significant alteration in cell viability, even at the highest concentration employed (0.1 %), which indicates that it did not interfere with the observed activity of compounds. Triton X-100TM (2 %), utilized as positive control, induced a mortality superior to 95%, confirming its utility in the assay. The MTT assay on RAW 264.7 macrophages showed that all of the tested FNQ produced reduction in cell viability proportional with increasing concentration of compounds, with no toxicity at 1 µM and percentages of cell viability below to 70 % at 10 µM, as can be seen in Figure 3.
Under our assay conditions, FNQ4 was the most toxic compound, LC50 value of 1.99 (1.46-2.88) ìM, followed by its linear conformer FNQ3, LC50 of 2.18 (1.71-2.80) ìM and compound FNQ5, LC50 of 7.25 (8.45-5.80 µM). FNQ1 and FNQ2 showed some toxicity at 10 µM producing 36 % and 49.5 % of cell death, respectively. The test compounds were considered to be cytotoxic when the percentage of cell survival was less than 80 %. Therefore FNQ1 and FNQ2 were evaluated in the assays of anti-inflammatory activity at concentrations lower than 5 ìM, and compounds FNQ3, FNQ4, and FNQ5 at concentrations lower than 1 µM.
INHIBITORY EFFECT OF FNQ ON NO• PRODUCTION
The induction of RAW 264.7 macrophages into an inflammatory state by treatment with LPS caused synthesis and release of NO•. Nitrite was detected in the medium at a mean concentration of 28.72 ± 7.18 µM. Cells that were not treated with LPS released trace amounts of NO•. 1 400W, a selective inhibitor of inducible nitric oxide synthase (iNOS), caused a decrease in LPS-induced NO production by more than 70 % at 10 µM.
FNQ1, FNQ2 and FNQ5 derivatives inhibited significantly NO• production in a concentration-dependent manner, with an IC50 value of 1.84, 1.04 and 0.54 µM, respectively, values lower than to the obtained for 1 400W, 3.72 (4.57-2.98 µM). Results of anti-inflammatory effect of tested compounds are summarized in Table. In addition, the efficacy of inhibition of NO• production was estimated calculating a selectivity index (SI). FNQ1, FNQ2 and FNQ5, showed SI higher than 5, which indicates their potential as safe anti-inflammatory drugs.28
NO-SCAVENGING EFFECT
SNP releases large amounts of NO• at physiological pH in aqueous solution. As expected, caffeic acid showed a significant effect as a scavenger of NO• (59.58 %). Results in Figure 4 indicated that the co-incubation of SNP with tested compounds at 10 µM did not diminished the levels of nitrite in the medium, indicating that suppression of NO• release shown by FNQ1, FNQ2 and FNQ5 can be directly attributed to blocking NO• production in LPS-stimulated RAW 264.7 macrophages.
DISCUSSION
Macrophages, named in this way because of their important role in phagocytosis of external intruders, antibody-antigen complexes and cellular debris, are fundamental in inflammatory and repair processes. They are considered the main immune effector cells, playing a pivotal role in the initiation, maintenance, and regulation of inflammation and innate immune response, the immediate arm of the immune system.29,30 During inflammation, macrophages are activated and produce reactive oxygen species (radical superoxide O2•- , hydrogen peroxide H2O2, the highly reactive hydroxyl radical •OH, and NO•), arachidonic acid metabolites, and lysosomal enzymes which are necessary to perform their phagocytic function.31
NO• is biosynthesis from the amino acid L-arginine in a process catalyzed by a family of oxidoreductases, called nitric oxide synthases (NOS). In NOS family, the inducible type (iNOS) is implicated in the pathophysiology of several chronic inflammatory conditions and can be activated in response to pro-inflammatory signals, such as cytokines and lipopolysaccharide (LPS) in various types of cells, including macrophages by binding TLR-4 receptor.32,33 NO• has multiple biological effects including vasodilatation and smooth muscle relaxation, inhibition of platelet aggregation, neuronal transmission, elimination of microorganisms and tumor cells, and it is recognized as an unique mediator of the inflammation and apoptosis. In small quantities, NO• acts protecting and repairing gastrointestinal mucosa, whereas abnormally high levels of this mediator has been described in a variety of pathological processes such as circulatory shock, carcinogenesis and chronic inflammation.34 Furthermore, the experimental data support the idea that compounds inhibiting overproduction of NO• are potential anti-inflammatory agents with beneficial effects.35 The purpose of this study was to evaluate the potential anti-inflammatory activity of synthetic analogues of FNQ isolated from the inner bark of species of the genus Tabebuia by determining NO• production by LPS-stimulated RAW 264.7 murine macrophages.
Quinones cyclization to FNQ derivatives, has allowed the preparation of compounds with a wide spectrum of biological activities. Therefore, this type of reactions is one of their most interesting characteristic, which has stimulated the development of several cyclization methods.36-40 Some of these reactions produced very low yields, situation that forced to find other alternatives. In our case, we employed reactions of cyclization of a variety of alkenes and alkynes in presence of lawsone. Reactions were mediated by CAN employing acetonitrile as solvent (Figure 1).41
The first precaution when studying the direct effect of a compound on NO• production is to guarantee that it does not cause cell death, decreasing the number of NO• producing cells. Thus, the effect on cell viability should be assessed.42 MTT assay revealed that all of the tested compounds produced toxic effect at 10 µM, being FNQ3 the most toxic one (Figure 3). The elevated toxicity of compounds was expected because, in general, tumor cells, as RAW 264.7 macrophages are sensitive to the effect of FNQ derivatives at low micromolar range.28,43 In addition, literature reports that compounds with FNQ moiety exhibits potent antiproliferative effect against several human neoplastic cell lines through three major mechanisms of toxicity: stimulation of oxidative stress, alkylation of cellular nucleophiles, and intercalation into DNA.44-47 Therefore, our findings suggest that FNQ4 is a promissory lead compound for the design, synthesis and development of new chemotherapeutics agents.
Whit regard to the NO• inhibitory activity of tested compounds, we found that FNQ1, FNQ2, and FNQ5 derivatives have a potent and selective inhibitory effect on NO• production in LPS-induced macrophages RAW 264.7 (Table), producing a mean IC50 value less than to that obtained for the positive control drug 1 400W, a highly selective inhibitor of iNOS, which is 5000 times more selective to this isoform than endothelial NOS (eNOS), and 200 times more than neuronal NOS (nNOS).48 This inhibitory effect might contribute significantly to the mechanism of anti-inflammatory activity reported for extracts and fractions obtained from species of Tabebuia.8,12,49 Also, this observation is in agreement with previous reports of anti-inflammatory activity of naphtoquinone derivatives which act inhibiting NO• production through down-regulation of iNOS expression by inactivating NF-kB.42,50,51 Based on previous experimental evidence, we strongly believe that NO• inhibitory activity of tested compounds is produced by blocking NF-êB activation, which is necessary for LPS induction of the iNOS promoter. The mechanisms by which FNQ derivatives could interfere with the activation of NF-êB remains to be elucidated.
Our results, positioned FNQ5 as the most active, CI50 0.54 (0.65-0.43 µM), and selective (SI= 13.77) compound. This observation might be related to its higher lipophilicity due to the presence of methyl substituents, which favors both the entrance of the compound in the cell and the establishment of hydrophobic bonds with a potential active site, leading to diverse biological effects, including selectivity among bioreceptors, increased potency, and protection against enzyme metabolism, etc.42,52 Thus, FNQ5 constitutes a promissory compound to investigate the in vivo effect and as a lead compound to design new derivatives which could suppress with greater potency and selectivity overproduction of NO•. On the other hand, we recommend further in vivo studies to establish the toxicity associated with the strong inhibition of NO• production of FNQ5, as well as other toxic side effect related to the naphthoquinone moiety.53-55
Upon comparing the structure-activity relationship of the tested compounds, it could generally be noted that modifications on furan ring substituents induce changes in the inhibition of NO• production and cytotoxic activity. Bulky aromatic substituents at 2-position of furan ring increased the cytotoxic activity (FNQ3 and FNQ4) and reduced the inhibition of NO• production. Whereas dimethyl groups (FNQ5) or vinyl groups (FNQ1 and FNQ2) at the same position, instead reduced the cytotoxicity (LC50 value higher than 7 µM) and led to a significant increase of inhibition of NO• activity (IC50 value lower than 1.8 µM). On the other hand, the small difference of FNQ1-FNQ2 and FNQ3-FNQ4 activities, suggest that linear (1,4-FNQ) or angular (1,2-FNQ) conformation, is not decisive for toxicity or NO• inhibitory activity (Figure 3 and Table).
CONCLUSION
In this work, we obtained six synthetic furanonaphthoquinones analogues of natural products isolated from Tabebuia genus, which exhibited a promissory profile. FNQ5 was the most active compound of the series, causing reduction of NO• production by LPS-stimulated RAW 264.7 murine macrophages, without cytotoxic or NO• radical scavengers effects, probably due to the presence of methyl substituents on the furan ring that rise the lipophilicity. The NO• inhibitory activity of the tested molecules varied with the type and nature of the substituents, which probably might affect their penetration and interaction with the potential target site. Furthermore, the slight difference in the activities of FNQ1 and FNQ2, suggests that linear or angular conformation is not determining for NO• inhibitory activity. To our knowledge, this is the first report showing the biological activity of these synthetic molecules and their potential either as an alternative therapy to treat inflammatory diseases related with NO• overproduction, or as lead compounds to design new anti-inflammatory drugs.
Acknowledments
This work was supported by the University of Cartagena, Cartagena, Colombia [grant number 053-2010] and Colciencias, Bogotá, Colombia [grant number 1107-04-16527]. Authors wish to thank the Young Investigators and Innovators Program «Virginia Gutiérrez de Pineda», from Colciencias, that sponsors Yanet Ocampo B.
REFERENCES
1. Huang G-J, Deng J-S, Liao J-C, Hou W-C, Wang S-Y, Sung P-J, et al. Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Participate in Anti-inflammatory Activity of Imperatorin from Glehnia littoralis. J Agric Food Chem. 2011;60(7):1673-81.
2. Ma L, Xie C, Ma Y, Liu J, Xiang M, Ye X, et al. Synthesis and biological evaluation of novel 5-benzylidenethiazolidine-2,4-dione derivatives for the treatment of inflammatory diseases. J Med Chem. 2011;54(7):2060-8.
3. Burk DR, Senechal-Willis P, Lopez LC, Hogue BG, Daskalova SM. Suppression of lipopolysaccharide-induced inflammatory responses in RAW 264.7 murine
macrophages by aqueous extract of Clinopodium vulgare L. (Lamiaceae). J Ethnopharmacol. 2009;126(3):397-405.
4. Conroy H, Mawhinney L, Donnelly S. Inflammation and cancer: macrophage migration inhibitory factor (MIF) the potential missing link. QJM. 2010;103(11):831.
5. Muangnoi C, Chingsuwanrote P, Praengamthanachoti P, Svasti S, Tuntipopipat S. Moringa oleifera Pod Inhibits Inflammatory Mediator Production by Lipopolysaccharide-Stimulated RAW 264.7 Murine Macrophage Cell Lines. Inflammation. 2012;35(2):445-55.
6. Justiniano M, Fredericksen, TS, Nash, D. Tajibos o lapachos Tabebuia spp. Gomes ex A.P. De Candolle Bignoniaceae. Bolivia: Proyecto de Manejo Forestal Sostenible (BOLFOR); 2000.
7. Warashina T, Nagatani Y, Noro T. Constituents from the bark of Tabebuia impetiginosa. Phytochemistry. 2004;65(13):2003-11.
8. Byeon SE, Chung JY, Lee YG, Kim BH, Kim KH, Cho JY. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J Ethnopharmacol. 2008;119(1):145-52.
9. Morais SKR, Silva SG, Portela CN, Nunomura SM, Quignard ELJ, Pohlit AM. Bioactive dihydroxyfuranonaphthoquinones from the bark of Tabebuia incana AH Gentry (Bignoniaceae) and HPLC analysis of commercial pau d'arco and certified T. incana bark infusions. Acta Amaz. 2007;37(1):99-102.
10. Yamashita M, Kaneko M, Tokuda H, Nishimura K, Kumeda Y, Iida A. Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant, Tabebuia avellanedae. Bioorg Med Chem. 2009;17(17):6286-91.
11. Kim BH, Lee J, Kim KH, Cho JY. Regulation of macrophage and monocyte immune responses by water extract from the inner bark of Tabebuia avellanedae. J Med Plant Res. 2010;4(6):431-8.
12. Franco LA, Castro JP, Ocampo YC, Pájaro IB, Díaz F. Actividad antiinflamatoria, antioxidante y antibacteriana de dos especies del género Tabebuia. Rev Cubana Plant Med. 2013;18(1). Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962013000100006&lng=es&nrm=iso
13. Girard M, Kindack D, Dawson BA, Ethier JC, Awang DVC, Gentry AH. Naphthoquinone constituents of Tabebuia spp. J Nat Prod. 1988;51(5):1023-4.
14. Rok Lee Y, So Kim B, Ug Jung Y, Soo Koh W, Soon Cha J, Woo Kim N. Facile synthesis of Avicequinone-B natural product. Synth Commun. 2002;32(20):3099-105.
15. Min-Hee K, Hyun-Mo S, Yong Rok L, Eun Yong C, Yoon Sook C, Kyung Rak M, et al. Suppressive effects of furonaphthoquinone NFD-37 on the production of lipopolysaccharide-inducible inflammatory mediators in macrophages RAW 264.7. Arch Pharm Res. 2005;28(10):1170-6.
16. Shin H-M, Lee YR, Chang YS, Lee J-Y, Kim BH, Min KR, et al. Suppression of interleukin-6 production in macrophages by furonaphthoquinone NFD-37. Int Immunopharmacol. 2006;6(6):916-23.
17. Wang JP, Chen YH, Kuo SC. Inhibition of hind-paw edema and cutaneous vascular plasma extravasation by 2-chloro-3-methoxycarbonylpropionamido-1,4-naphthoquinone (PP1D1) in mice. Naunyn Schmiedebergs Arch Pharmacol. 1996;354(6):779-84.
18. Tanaka S, Nishiumi S, Nishida M, Mizushina Y, Kobayashi K, Masuda A, et al. Vitamin K3 attenuates lipopolysaccharide-induced acute lung injury through inhibition of nuclear factor-êB activation. Clin Exp Immunol. 2010;160(2):283-92.
19. Díaz F, Medina JD. Furanonaphthoquinones from Tabebuia ochracea ssp. neochrysanta. J Nat Prod. 1996;59(4):423-4.
20. Gaitán R, Marrugo J, Franco LA, Gómez HA, Mercado JE. Componentes del género Tabebuia como inhibidores de la linfoproliferación. Biotecnología en el Sector Agropecuario y Agroindustrial. 2009;1:149-50.
21. Pérez H, Díaz F, Medina JD. Chemical Investigation and in vitro Antimalarial Activity of Tabebuia ochracea ssp. neochrysantha. Pharm Biol. 1997;35(4):227-31.
22. Kobayashi K, Uneda T, Tanaka K, Mori M, Tanaka H, Morikawa O, et al. One-step synthesis of naphthofurandione, benzofurandione, and phenalenofuranone derivatives by the CAN-mediated cycloaddition. Bull Chem Soc Jpn. 1998;71(7):1691-7.
23. Lee YR, Kim BS, Kim DH. Ceric Ammonium Nitrate (CAN)-Mediated Oxidative Cycloaddition of 1, 3-Dicarbonyls to Conjugated Compounds. Efficient Synthesis of Dihydrofurans, Dihydrofurocoumarins, Dihydrofuroquinolinones, Dihydrofurophenalenones, and Furonaphthoquinone Natural Products. Tetrahedron. 2000;56(45):8845-53.
24. Gaitan R, Arguello E, Álvarez W, Jaraba S. Obtención de análogos de productos naturales furanonaftoquinonicos y evaluación de su actividad antimalarica frente a Plasmodium falciparum. Scientia Et Technica. 2007(33):141-4.
25. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63.
26. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal Biochem. 1982;126(1):131-8.
27. de la Puerta Ro, Domýìnguez MEMn, Ruýìz-Gutýìerrez V, Flavill JA, Hoult JRS. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001;69(10):1213-22.
28. Nishishiro M, Arikawa S, Wakabayashi H, Hashimoto K, Satoh K, Yokoyama K, et al. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by azulenequinones. Anticancer Res. 2005;25(6B):4157-63.
29. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of Monocytes, Macrophages, and Dendritic Cells. Science. 2010;327(5966):656-61.
30. Park JW, Kwon OK, Jang H, Jeong H, Oh SR, Lee HK, et al. A Leaf Methanolic Extract of Wercklea insignis Attenuates the Lipopolysaccharide-Induced Inflammatory Response by Blocking the NF-êB Signaling Pathway in RAW 264.7 Macrophages. Inflammation. 2012;35(1):321-31.
31. Heo SJ, Yoon WJ, Kim KN, Ahn GN, Kang SM, Kang DH, et al. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem Toxicol. 2010;48(8-9):2045-51.
32. Ren J, Chung SH. Anti-inflammatory effect of á-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-êB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55(13):5073-80.
33. Nakamura T, Kodama N, Arai Y, Kumamoto T, Higuchi Y, Chaichantipyuth C, et al. Inhibitory effect of oxycoumarins isolated from the Thai medicinal plant Clausena guillauminii on the inflammation mediators, iNOS, TNF-a, and COX-2 expression in mouse macrophage RAW 264.7. Journal of natural medicines. 2009;63(1):21-7.
34. Cirino G. Multiple Controls in Inflammation: Extracellular and Intracellular Phospholipase A2, Inducible and Constitutive Cyclooxygenase, and Inducible Nitric Oxide Synthase. Biochem Pharmacol. 1998;55(2):105-11.
35. Hamalainen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:456-73.
36. Huot R, Brassard P. Synthèse de méthyl-3 furoquinones. Can J Chem. 1974;52(1):88-94.
37. Hagiwara H, Sato K, Suzuki T, Ando M. Tandem Nucleophilic Reaction Leading to Hydrofurans: Application to One-Pot Synthesis of Antitumor Naphthoturan Natural Product. Heterocycles. 1999;51(3):497-500.
38. Kobayashi K, Shimizu H, Sasaki A, Suginome H. Photoinduced molecular transformations. 140. New one-step general synthesis of naphtho [2, 3-b] furan-4, 9-diones and their 2, 3-dihydro derivatives by the regioselective [3+ 2] photoaddition of 2-hydroxy-1, 4-naphthoquinones with various alkynes and alkenes: Application of the photoaddition to a two-step synthesis of maturinone. J Org Chem. 1993;58(17):4614-8.
39. Kobayashi K, Kanno Y, Suginome H. Photoinduced molecular transformations. Part 141. New one-step general synthesis of benzofuran-4,7-diones by the regioselective (3 + 2) photoaddition of 2-hydroxy-1,4-benzoquinones with various alkenes. J Chem Soc, Perkin Trans 1. 1993(13):1449-52.
40. Chuang CP, Wang SF. Manganese (III) acetate initiated oxidative free radical reaction between 1, 4-naphthoquinones and [alpha]-alkylmalonates. Tetrahedron. 1998;54(34):10043-52.
41. Suginome H, Konishi A, Sakurai H, Minakawa H, Takeda T, Senboku H, et al. Photoinduced molecular transformations. Part 156. New photoadditions of 2-hydroxy-1,4-naphthoquinones with naphthols and their derivatiyes. Tetrahedron. 1995;51(5):1377-86.
42. Pinho BR, Sousa C, Valentao P, Andrade PB. Is nitric oxide decrease observed with naphthoquinones in LPS stimulated RAW 264.7 macrophages a beneficial property? PLoS One. 2011;6(8):e24098.
43. Miguel del Corral JM, Castro MA, Oliveira AB, Gualberto SA, Cuevas C, San Feliciano A. New cytotoxic furoquinones obtained from terpenyl-1,4-naphthoquinones and 1,4-anthracenediones. Bioorg Med Chem. 2006;14(21):7231-40.
44. Jimenez-Alonso S, Guasch J, Estevez-Braun A, Ratera I, Veciana J, Ravelo AG. Electronic and cytotoxic properties of 2-amino-naphtho[2,3-b]furan-4,9-diones. J Org Chem. 2011;76(6):1634-43.
45. Su JC, Lin KL, Chien CM, Tseng CH, Chen YL, Chang LS, et al. Naphtho[1,2-b]furan-4,5-dione inactivates EGFR and PI3K/Akt signaling pathways in human lung adenocarcinoma A549 cells. Life Sci. 2010;86(5-6):207-13.
46. Eyong KO, Kumar PS, Kuete V, Folefoc GN, Nkengfack EA, Baskaran S. Semisynthesis and antitumoral activity of 2-acetylfuranonaphthoquinone and other naphthoquinone derivatives from lapachol. Bioorg Med Chem Lett. 2008;18(20):5387-90.
47. Desmond JC, Kawabata H, Mueller-Tidow C, Simamura E, Heber D, Hirai K, et al. The synthetic furanonaphthoquinone induces growth arrest, apoptosis and differentiation in a variety of leukaemias and multiple myeloma cells. Br J Haematol. 2005;131(4):520-9.
48. Chiaradia LD, dos Santos R, Vitor CE, Vieira AA, Leal PC, Nunes RJ, et al. Synthesis and pharmacological activity of chalcones derived from 2,4,6-trimethoxyacetophenone in RAW 264.7 cells stimulated by LPS: quantitative structure-activity relationships. Bioorg Med Chem. 2008;16(2):658-67.
49. Kreher B, Lotter H, Cordell GA, Wagner H. New Furanonaphthoquinones and other Constituents of Tabebuia avellanedae and their Immunomodulating Activities in vitro. Planta Med. 1988;54(6):562-3.
50. Tseng CH, Lin CS, Shih PK, Tsao LT, Wang JP, Cheng CM, et al. Furo[3',2':3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg Med Chem. 2009;17(18):6773-9.
51. Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH, Kang JJ. Shikonin derivatives inhibited LPS-induced NOS in RAW 264.7 cells via downregulation of MAPK/NF-kB signaling. J Ethnopharmacol. 2008;120(2):264-71.
52. Barreiro EJ, Kummerle AE, Fraga CAM. The Methylation Effect in Medicinal Chemistry. Chem Rev. 2011;111(9):5215-46.
53. Medeiros CS, Pontes-Filho NT, Camara CA, Lima-Filho JV, Oliveira PC, Lemos SA, et al. Antifungal activity of the naphthoquinone beta-lapachone against disseminated infection with Cryptococcus neoformans var. neoformans in dexamethasone-immunosuppressed Swiss mice. Braz J Med Biol Res. 2010;43:345-9.
54. de Cássia R, de Oliveira M. Reproductive toxicity of lapachol in adult male Wistar rats submitted to short-term treatment. Phytother Res. 2007;21(7):658-62.
55. Maistro EL, Fernandes DM, Pereira FM, Andrade SF. Lapachol Induces Clastogenic Effects in Rats. Planta Med. 2010;76:858-62.
Recibido: 8 de julio de 2013.
Aprobado: 22 de agosto de 2013.
Luis Alberto Franco Ospina. Faculty of Pharmaceutical Sciences, University of Cartagena. Cartagena, 130015, Colombia. Phone: +57-5-6698323, Fax No.: +57-5-6698278