Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Medicina Militar
versión impresa ISSN 0138-6557versión On-line ISSN 1561-3046
Rev Cub Med Mil vol.49 no.3 Ciudad de la Habana jul.-set. 2020 Epub 25-Nov-2020
Presentación de tecnología
A modified method for synthesis of amantadine hydrochloride
1Vietnam Military Medical University. Hanoi, Vietnam.
2The R&D department, 3B6 chemical service and production joint-stock company. Hanoi, Vietnam.
3School of Chemical Engineering, Hanoi University of Science and Technology. Hanoi, Vietnam.
4Universidad de Ciencias Médicas de las Fuerzas Armadas Revolucionarias. La Habana, Cuba.
Introduction:
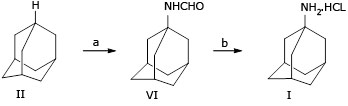
Amantadine hydrochloride (I) was well-known as an antiviral drug used to prevent and treat influenza A infections. Besides, it also was used to relieve the symptoms of Parkinson's disease in the early period. Several methods for the preparation of I have been reported. These procedures started with adamantane (II) in four or three reaction steps to produce amantadine hydrochloride with overall yields ranging from 45% to 58%.
Objectives:
Improving method for synthesis of amantadine hydrochloride could introduce to industrial scale.
Methods:
Step-by-step optimization to reduce the use of reagents, solvents, as well as the conditions of each step were screened to be milder and more environment-friendly.
Results:
All factors related to the yield of reaction to synthesize the intermediate and final compounds were screened to give the highest yield of each step. Finally, a two-step procedure for the synthesis of (I) from (II) via N-(1-adamantyl) formamide (III) with improving overall yield of 78% and a purity of 99.2% was established, and the structure of the product was confirmed by 1H-NMR,13C-NMR, IR and MS. The synthesis of N-(1-adamantyl) formamide (VI) from (II) also was successfully accomplished within only one step. This method avoided the use of liquid bromine or fuming sulfuric acid as reactants. The subsequent conversion of (VI) to (I) was carried out under milder reaction conditions without using hazardous solvents.
Conclusions:
An improved synthesis for amantadine hydrochloride (I) have been provided. This research can be an industrially convenient production of amantadine hydrochloride. Because the raw materials and reagents used in this research are cheap and available which also were screened to save their use. Moreover, the total preparation time was significantly reduced to save energy as well as labor.
Keyword: amantadine hydrochloride; adamantine; N-(1-adamantyl) acetamide; antiviral; antidyskinetic
INTRODUCTION
Amantadine hydrochloride (I) (tricycle[3.3.1.1(3,7)] decan-1-amine hydrochloride) drug and its antiviral activity was discovered in 1964 by Davies W. L. et al.1 Later, amantadine hydrochloride was studied and applied to treat many diseases like Parkinson's disease in early symptoms period, and dyskinesia. It was approved by the United States Food and Drug Administration in 19662) and has been used to treat influenza A infection in the Asian influenza epidemic.
Continuously, the modifying and improving methods of synthesis of amantadine and amantadine hydrochloride (I) were reported using one of the following raw materials: adamantane (II),3,4,5,6,7 1-bromoadamantane (III),8,9,10 1-adamantanecarboxylic acid,11,12,13) 1-adamantanol,14,15) 1-adamantyl-magnesium-bromide,16,17,18) and tetrahydrodicyclopentadiene.19
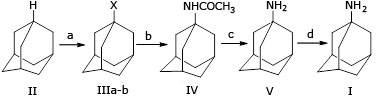
Many studies have reported the synthesis of (II), produces 1-bromoadamantane (IIIa) or 1-adamantyl nitrate (IIIb), respectively. However, in the conversion step of (IIIa) or (IIIb) to N -(1-adamantyl) acetylamide (IV), they used the concentrated sulfuric acid and acetonitrile, and then treated with sodium hydroxide in polyethylene glycol (PEG) at reflux conditions (240-250 oC) to yield amantadine (V). Finally, in the salt formation of (V) was used anhydrous HCl in ether solution to produce (I) relatively low overall yields between 45% to 58% (Fig.1).20,21
From those studies, to produce amantadine hydrochloride (I) in industrial production, several issues should be addressed. First, the bromination step was carried out under reflux, which can emit poisonous bromine vapor. Second, 1-bromoadamantane (IIIa) is sensitive to moisture, and thus not suitable for long-term storage.22) Third, the concern of the use of solvents, chemicals, and the temperature issue, because benzene and diethylene glycol (DEG) are very toxic at high temperature.20,21 Especially, DEG can also decompose exothermally when heated to 230 oC, releasing explosive hydrogen gas and acid smoke.22 Finally, using ether as a solvent for amantadine extraction and subsequent hydrochloride formation with anhydrous HCl in ether is a matter of concern because of its highly flammable and forming to peroxides.20,21,23,24
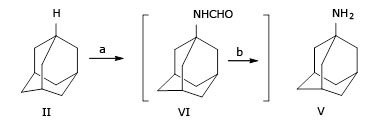
Although Wolfgang H. has described the synthesis of amantadine (V) from (II) via two steps in one-pot, whereby formamidation of (II) in n-hexane with a mixture of liquid hydrocyanic acid and tertiary- butanol in the presence of sulfuric acid at 25 oC/2.0 h to give (VI) with a molar ratio between (sulfuric acid 96% : adamantine : n-hexane : liquid hydrocyanic acid : t-butanol) = (65 : 1 : 1.17 : 17 : 4). And then hydrolyzed (VI) by treatment with sodium hydroxide and diethylene glycol (DEG) at reflux conditions (245 - 259 oC for 10 h), the product V was isolated from the reaction mixture by ethyl ether extraction and gives amantadine (V) with yield 70% (Fig. 2):23
Nevertheless, the above procedure still has several disadvantages, including a) liquid hydrocyanic is a rare reagent and toxic; b) the molar ratio of reagents among sulfuric acid 96% : (II) : n-hexane : liquid hydrocyanic acid : t-butanol) = (65 : 1 : 1.17 : 17 : 4) indicates that sulfuric acid and liquid hydrocyanic acid are used lavishly; c) ether as a solvent for extraction of amantadine is a matter of concern because it its highly flammable.
In this article, we report a modified method for the synthesis of amantadine hydrochloride from adamantane in two steps separately or within one-pot in higher relative overall yield. Each step was conditions optimized which effected on the yield of intermediate and final product. Besides, avoiding the use of sulfuric acid and liquid hydrocyanic acid also is a solution to protect the environment because they cause pollution in industrial production.
METHODS
To establish the suitable reaction conditions which can apply to industrial scale, we have tried to screen all factors with effects on the yield of amantadine hydrochloride.
1. Effect of reaction parameters on the yield of N-(1-adamantyl) formamide (VI)
1.1. Effect of reaction temperature on the yield of (VI)
Experiment: To nitric acid (5 mL, 0.12 mol), adamantane (II) (1.4 g, 0.01 mol) was added for 15 minutes at 25-30 oC and then to this mixture potassium cyanide (1.47 g, 0.02 mol) was added in portion with stirring at 25-30 oC over 10 minutes. The reaction mixture was stirred at 40-45 oC, 50-55 oC, 50-60 oC, 60-65 oC, 65-70 oC, 70-75 oC for reaction to finish (the end of reaction was indicated by thin layer chromatography (TLC); solvent: mixture of n-hexane and acetone = 4:1). At the end of the reaction, the reaction mixture was added to ice water (15 mL) and stirred for 1.0 h at 0-5 oC. The white solid was precipitated, filtered, and washed with cool water. The obtained product was dried under vacuum to yield N-(1-adamantyl)formamide (VI) (table 1).
Table 1 Effect of reaction temperature and reaction time on the yield of N-(1-adamantyl) formamide (VI)
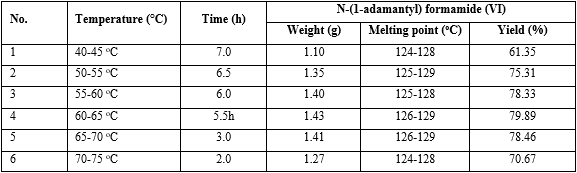
The optimal reaction temperature was 60-65 oC and reaction time was 5.5 h (see No. 4 in table 1)
Experiment: The reaction synthesis of N-(1-adamantyl) formamide (VI) was performed with the same operation as investigation on the effect of the reaction temperature and reaction term on the yield of (VI) above, but reaction temperature was 60-65oC, reaction time 5.5 h and indifferent the molar ratio between nitric acid and adamantane from 10:1 to 16:1 (table 2).
Table 2 Effect of molar ratio between nitric acid and adamantane on the yield of N-(1-adamantyl)formamide (VI)
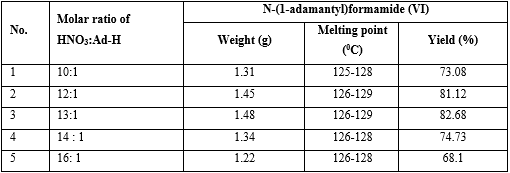
The result found that using molar ratio of (potassium cyanide : adamantane) = (13 : 1) got the highest yield of N-(1-adamantyl) formamide (VI) (see No. 3 in table 2).
Experiment: The reaction synthesis of (VI) was performed with the same operation as investigation on the effect of molar ratio between nitric acid and adamantane on the yield of N-(1-adamantyl)formamide (VI) above, but the molar ratio of nitric acid adamantine was 13:1 and indifferent the molar ratio between potassium cyanide and adamantine was 1.25:1; 1.5:1; 1.75:1; 2.0:1 and 2.0:1 (table 3).
Table 3 Effect of molar ratio between potassium cyanide and adamantane on the yield of N-(1-adamantyl) formamide (VI)

The result found that using a molar ratio of (potassium cyanide: adamantane) = (1.5: 1) got the highest yield of N-(1-adamantyl) formamide (VI) (see No. 2 in table 3).
To give highest yield of N-(1-adamantyl) formamide (VI), the combination of reaction parameters was: Temperature = 60-65 °C; Time = 5.5 h; Molar ratio of (nitric acid: potassium cyanide: adamantane) = (13:1.5:1).
2. Effect of reaction parameters on the synthesis of amantadine.HCl (I)
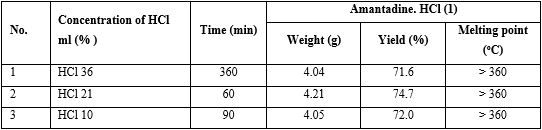
2.1. Effect of concentration of HCl on the yield of (I)
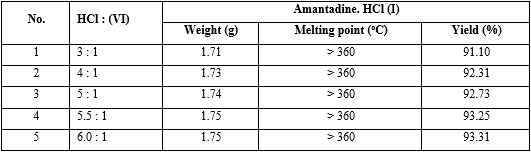
A mixture of a solution of hydrochloride (0.09 mol) in different the concentration (36%, 21%, 10%) and N-formyl-1-amino-adamantane (VI) (5.40 g, 0.03 mol) was stirred at room temperature for 10 min, and then it was heated to reflux for until reaction finished off (the end of reaction was indicated by TLC; solvent: a mixture of chloroform : methanol : 25% NH 3 aq. = 6:1:1). Then the reaction mixture was concentrated to dryness in a vacuum, to which this reaction mass ethyl acetate (8 mL) was added, the reaction mixture was heated to reflux for 0.5 h and then at 5-10 oC over 1 h, the white solid was precipitated, filtered and washed with cooled ethyl acetate and dried under vacuum to give 1-adamantylamine hydrochloride (I), mp > 360 oC (not melting from ethanol), Rf = 0,5 (CHCl3: methanol : 25%NH3 aq. = 6 : 1 : 1) (table 4).
The optimal concentration of HCl was 21%, and the reaction time was 60 min (see No. 2 in table 4).
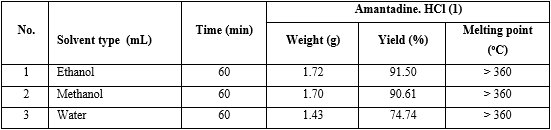
Experiment: The reaction synthesis of (I) was performed with the same operation as an investigation on the effect of concentration of HCl on the yield of amantadine.HCl (I) above, indifferent solvents were water, ethanol, methanol (table 5).
The results found that using ethanol as solvent got the highest yield of amantadine.HCl (I) (see No. 1 in table 5).
Experiment: The reaction synthesis of (I) was performed with the same operation as an investigation on the effect of concentration of HCl on the yield of amantadine.HCl (I) above, indifferent molar ratio between HCl and N-(1-adamantyl)formamide (VI) 3:1, 4:1, 5:1, 5.5:1, 6:1 (table 6).
The combination of reaction parameters that gives the highest yield of amantadine.HCl is: Temperature = 80-85 °C; Time = 60 minutes; Molar ratio of (HCl: N-(1-adamantyl)formamide = (5.5 : 1); Ratio of Solution HCl % and Ethanol = 1 : 1 (V/V).
RESULTS
Based on the optimal conditions, we applied to synthesize the intermediate and final compound.
N-(1-adamantyl) formamide (VI):
Adamantane (II) (5.51 g, 0.04 mol) was added to nitric acid (22 mL, 0.52 mol) with stirring at 25-30 oC, for 10 minutes and then to this mixture potassium cyanide (3.95 g, 0.06 mol) was added in portion with stirring at 25-30oC over 20 minutes. The reaction mixture was stirred at 60-65 oC over 5.5 h. At the end of the reaction, ice water (30 mL) was added to the reaction mixture with stirring over 1.0 h at 0-5 oC. The white solid was precipitated, filtered and washed with cool water. The obtained product was dried under vacuum to yield N-(1-adamantyl) formamide (VI). Yield: 5.94 g (82.85%) . Purity (GC): 99.20%, tR 15.90 min; m.p. 125-129 oC. MS, m/z: 180.26 [M+1]+; 166.40; 158.96; 1H-NMR (400 MHz, CDCl3), δ (ppm): 8.27 (d, J=12.3Hz, 0.67H,); 8.03 (d, J= 1.7Hz, 0.33H); 5.82 (s, 0.65H); 5.132 (s, 0.35H); 2.10 (d, J=18.6 Hz, 3H), 1.84 (d, J= 2.4Hz, 2H),; 1.74 (d, J= 2.2Hz, 4H), 1.69-1.65 (m, 6H); 13C -NMR(100 MHz, CDCl 3), δ (ppm): 162.28; 160.30; 52.09; 50.65; 44.11; 41.83; 36.2; 35.80; 29.30; 29.28.
Amantadine hydrochloride (I):
A mixture of N-(1-adamantyl) formamide (VI) (5.42 g, 0.03 mol), ethanol (13 mL) and concentrated HCl 36% (13 mL, 0.165 mol) was stirred at 80-85 oC over 1 h, then evaporated under vacuum to give a white solid. Ethyl acetate (8 mL) was added, stirred at 50 oC over 0.5 h, and then at 0-5 oC for additional 1 h. The product is a white solid which was filtered and dried under vacuum to give (I). Yield: 5.27 g (93.68%). R f = 0.5 (CHCl3/MeOH/25% aquous NH3= 6:1:1). Purity (GC): 99.22%, tR10.10 min; mp 360 oC. MS, m/z: 151.9[M+1]+; 134.9 [M-NH2]+; 1H-NMR (500 MHz, CDCl3), δ (ppm): 8.28 (br, s, 3H, NH2.HCl); 2,15 (s, 3H, C3-H, C5-H, C7-H); 2.04 (s, 6H, C4-H2,C6-H2, C9-H2);1.69 (s, 6H, C2-H2, C8-H2, C10-H2);13C-NMR (125 MHz, CDCl3), δ (ppm): 52.9 (C 1); 40.6 (C3+C5 and C7); 35.4 (C2+C8 and C10); 29.0 (C4+C 6 and C9).
Synthesis in situ of Amantadine hydrochloride (I) from Adamantane:
Adamantane (138 g, 1.0 mol) was added to nitric acid (550 mL, 13 mol) with stirring at 25-30 oC over 30 min and then to this mixture potassium cyanide (99 g, 1.5 mol) was added in portion with stirring at 25-30 oC over 40 min. The reaction mixture was stirred at 60-65 oC for 5.5 h. At the end of the reaction, ice water (700 mL) was added to the reaction mixture and stirred for 1.0 h at 0-5 oC. The white solid was precipitated, filtered, and washed with cool water. The obtained product was added to a mixture of methanol (430 mL) and solution of 36% hydrochloride (430 mL, 5.5 mol) and stirred at room temperature for 10 min, and then it was heated to reflux (80-85oC) for 1 h, then evaporated under vacuum to give a white solid, to which was added ethyl acetate (200 mL), stirred at 50 oC for 0.5 h, and then at 0-5 oC for additional 1 h. The final product was collected by filtering and drying under vacuum to give (I). Yield: 146 g (77.75% ). Rf = 0.5 (CHCl3/MeOH/25% aqueous NH3= 6:1:1).
DISCUSSION
In our research, compound (VI) was identified as a suitable intermediate to prepare (I) via a one-step from (II): Direct conversion of (II) to (VI) is the key step in the synthesis of (I). The formamidation of (II) with nitric acid and potassium cyanide was stirred at 60-65 oC over 5.5 hrs, and the product was poured on ice, to give (VI). The conversion of (VI) to (I) was carried out under milder conditions without using hazardous solvent (in a solution of 36% HCl and 96% ethanol at 80-85 oC yields (I)), the overall yield of 78% (Fig. 3).
Synthesis of N-(1-adamantyl) formamide (VI). Compound (VI) was prepared from (II) in one step via the Ritter reaction. In this reaction, nitric acid induced the addition of a nitril (−C≡N) to the carbenium ion of (II), which on treatment with water produces (VI). This method bypassed the bromination or nitration of (II) (Fig. 1, step a), thus eliminating the need for liquid bromine or fuming nitric acid (Fig. 1, step a) or not to use liquid hydrocyanic acid and not using too much sulfuric acid (Fig. 2). In addition, the reaction temperature and reaction time (see table 1) and the molar ratio between reactants (table 2, table 3) were optimized to reduce the use of nitric acid. The result show that reaction carry out at 60-65 oC over 5,5 h, the molar ratio of (adamantine II : nitric acid : potassium cyanide) was 1:13:1.5 (instead molar ratio of adamantine : sulfuric acid: liquid hydrocyanic acid : t-butanol was 65:1:17:4 in a previous report23). Finally, water was used as the alternative solvent to benzene for isolation and separation of (VI). This change reduced the toxicity level of the described procedure.
Synthesis of amantadine hydrochloride (I). Conversion of (VI) into (I) under milder conditions and shorter reaction time without using a hazardous solvent. This hydrolysis reaction was carried out in a mixture of a solution of HCl and ethanol on 80-85 oC over 1 h instead of sodium hydroxide in diethylene glycol (DEG) on reflux 245-250 oC over 15 h (in Fig. 1) or sodium hydroxide in diethylene glycol (DEG) on reflux for 10 h (in Fig. 2). The HCl concentration, the solvent type for defomylation of (VI) into (I) and molar ratio between HCl and (VI) was also optimized for deformylation (see table 4, table 5, table 6), which resulted in a lower reaction temperature (80-85 oC) and a shorter reaction time (1 h) than described previously (i.e., 240-245 oC; 10 -15 h).20,21,23
In summary, Fig. 3 presents a safe, economically competitive friendly synthesis of (I), which is suitable for work at a hectogram scale. Compound (I) was obtained in two steps or with two-step in one-pot, with a high overall yield of 78% (compared to overall yields of 45-58% in four steps20,21).
REFERENCIAS BIBLIOGRÁFICOS
1. Davies WL, Grunert RR, Haff RF, McGahen JW, Neumayer EM, Paulshock M, et al. Antiviral Activity of 1-Adamantanamine (Amantadine). Science. 1964[acceso: 23/08/2015]; 144(3620): 862-3. Available from: Available from: https://science.sciencemag.org/content/144/3620/862/tab-pdf [ Links ]
2 . Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology. 2012[acceso: 02/09/2015]; 78(14):1096-99. Available from: Available from: https://n.neurology.org/content/78/14/1096 [ Links ]
3 . Kojima A, Ito K, Kono N, inventor; Method for production of amines and quaternary ammonium salt with adamantane skeleton. Japan Patent JP 2011-051976A. 17 Mar 2011. [ Links ]
4 . He JX, Wang HB, Zhou HY. Synthesis of Amantadine Hydrochloride. Chinese Journal of Pharmaceuticals. 2013[acceso: 12/12/2015]; 44(1): 1-3. Available from: Available from: http://www.cjph.com.cn/EN/volumn/volumn_1150.shtml# [ Links ]
5 . Qiu Y, Li L, Wang J, Wu , Lin Z, Chen X, inventor; Adamantanamine hydrochloride preparation method. China Patent CN 102875387A. 16 Jan 2013. [ Links ]
6 . Vu BD, Nguyen VT, Le TS, Phan DC. An Improved Synthsis of Amantadine Hydrochloride. Org. Process Res. Dev. 2017[acceso: 28/09/2017]; 21(11):1758-60. Available from: Available from: https://pubs.acs.org/doi/abs/10.1021/acs.oprd.7b00242 [ Links ]
7 . Thinh NV, Pham VH, Vu BD, Dang TA, Tran KV, Phan DC. Mirowave Method for Synthesis of Amantadine Hydrochloride. Chang Mai J. Sci. 2018[acceso: 01/10/2018]; 45(6): 2454-58. Available from: Available from: https://epg.science.cmu.ac.th/ejournal/journalDetail.php?journal_id=9530 [ Links ]
8 .Marvin P, Watts JC, inventor; EI Du Pont Nemours and Co., assignee. Pharmaceutical compositions and methods utilizing 1-aminoadamantane and its derivatives. United State Patent US 3,310,469. 21 Mar 1967. [ Links ]
9 . Kraus GA, inventor; Iowa State University Research Foundation (ISURF), assignee. Method for the synthesis of adamantane amines United State Patent US 5,599,998. 4 Feb 1997. [ Links ]
10. Shao G, Yang M, Wu C. Synthesis of amantadine hydrochloride. Chemical Intermediate, 2009[acceso: 15/10/2017]; 7: 55-56. Available from: Available from: https://caod.oriprobe.com/articles/16554366/Synthesis_of_-Amantadine_Hydrochloride.htm [ Links ]
11 . Vincent CW, Bruce AR, inventor; Wyeth Holding LLC, assignee. Method of preparing 1-adamantanamine. United State Patent US 3,388,164. 11 Jun 1968. [ Links ]
12 . Eriks K, Jack M, inventor; Eli Lilly and Co., assignee. Adamantyl secondary amines. United State Patent US 3,391,142 A. 2 Jul 1968. [ Links ]
13 . Butov GM, Pershin VV, Burmistrov VV, inventor; State educational institution of higher professional education Volgograd State Technical University (VolgSTU), assignee. Method of producing hydrochlorides of amine-derivatives of adamantine. Russia patent RU2440971C1. 27 Jan 2012. [ Links ]
14 . Jirgensons A, Kauss V, Kalvinsh I, Gold MR. A Practical Synthesis of tert-Alkylamines via the Ritter Reaction with Chloroacetonitrile. Synthesis. 2000. [acceso: 15/01/2016]; 2000(12):1709-12. Available from: Available from: https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-2000-8208.pdf [ Links ]
15 . Schickaneder CP, inventor; Hexal AG., assignee. Process for the Preparation of Adamantanamines. United State Patent US 20090082596A1. 26 Mar 2009. [ Links ]
16 . Tsutsui H, Ichikawa T, Narasaka K. Preparation of Primary Amines by the Alkylation of O-Sulfonyloximes of Benzophenone Derivatives with Grignard Reagents. Bull. Chem.Soc. Jpn. 1999[acceso: 25/05/2016]; 72(8): 1869-78. Available from: Available from: https://www.journal.csj.jp/doi/pdf/10.1246/bcsj.72.1869 [ Links ]
17 . Kitamura M, Chiba S, Narasaka K. Synthesis of primary amines and N-methylamines by the electrophilic amination of grignard reagents with 2-imidazolidinone O-sulfonyloxime. Bull. Chem.Soc. Jpn. 2003[acceso: 22/12/2015] ; 76(5): 1063-70. Available from : Available from : https://www.journal.csj.jp/doi/pdf/10.1246/bcsj.76.1063 [ Links ]
18 . Kitamura M, Suga T, Chiba S, Narasaka K. Synthesis of Primary Amines by the Electrophilic Amination of Grignard Reagents with 1,3-Dioxolan-2-one O-Sulfonyloxime. Org. Lett. 2004[acceso: 20/10/2016] ; 6(24): 4619-21. Available from: Available from: https://pubs.acs.org/doi/pdf/10.1021/ol0479951 [ Links ]
19 . Zhang Z, inventor; Synthesis method for Amantadine Hydrochloride. China Patent CN 102050744B. 30 Nov 2011. [ Links ]
20 . Stetter H, Mayer J, Schwarz M, Wulff K, Über Verbindungen mit Urotropin-Struktur, XVI. Beiträge zur Chemie der Adamantyl-(1)-Derivate. Eur. J. Inorg. Chem. 1960[acceso: 10/10/2015]. 93(1):226-30. Available from: Available from: https://chemistry-europe.onlinelibrary.wiley.com/doi/epdf/10.1002/cber.19600930133 [ Links ]
21 . Moiseev IK, Doroshenko RI, Ivanova VI. Synthesis of amantadine through medium of nitrate 1-adamantanol. Pharm. Chem. J. 1976[acceso: 22/08/2015]; 10:450-51. Available from: Available from: https://link.springer.com/article/10.1007%2FBF00757832 [ Links ]
22 . Periandy N, Kilaru S, Thennati R, inventor; Novel process for the preparation of aminoadamantane derivatives. World Intellectual Property Organization Patent WO2005062724 A2. 14 Jul 2005. [ Links ]
23 . Wolfgang H, inventor; Studiengesellschaft Kohle mbH, assignee. Process for the production of N-tert-alkyl amides and, if desired, N-tert. alkyl amines. United State patent US 3,152,180. 6 Oct. 1964. [ Links ]
24 . Yao F, Xie L, Guo YF, inventor; Synthetic method of adamantanamine hydrochloride. China Patents CN105294445A. 3 Feb 2016. [ Links ]
Received: April 09, 2020; Accepted: July 20, 2020











 texto en
texto en