INTRODUCTION
The success of frozen embryo transfer (FET) cycles depends on a delicate interaction between embryo quality and endometrium.1,2 Endometrium changes immediately after ovulation in order to receive embryo implantation. Endometrial tissue contains growth factor receptors, adhesion molecules, cytokines, growth hormone, platelet-derived growth factor (PDGF), epithelial growth factor (EGF), and lipids that promote proliferation of the endometrium, secretion of glands, blood vessels and connective cells of stroma.3 The endometrial thickness is a crucial criterion in FET cycles.
Thin endometrium in assisted reproduction is often defined as endometrial thickness being less than 7 mm or 8 mm.4,5,6 Although pregnancy results are reported in both 4 and 5 mm thick endometrium, low pregnancy success rates are associated with a thin endometrium.7,8 In 2009, Kumbak et al.8 compared clinical pregnancy rates between the group with thin endometrium (7 mm; 175 patients) and the group with endometrium thickness ≥ 7 mm (5 573 patients). The clinical pregnancy rates were 26 % and 51 %, respectively (p< 0.0001). El-Toukhy et al.9 performed 768 FET cycles, which included 25 cycles with endometrium thickness < 7 mm and > 14 mm, 357 cycles with the endometrium thickness between 7 - 8 mm, 386 cycles with the endometrium thickness between 9 - 14 mm, the clinical pregnancy rates were 7 %, 18 %, and 30 %, respectively. The difference between the 7 - 8 mm group and the 9 -14 mm group was significant with P< 0.001, the group with endometrium < 7mm and > 14 mm was not compared with the other groups due to the small quantity and the extremes of endometrial thickness might be a confounding factor.9
Several treatments were used to improve the endometrial thickness in FET cycles, including extended estrogen administration,10 low-dose aspirin,11 vitamin E, vasodilators such as vaginal sildenafil, oral pentoxifylline, nitroglycerin patches, L-arginine,12,13 and intrauterine infusion of granulocyte colony-stimulating factor (G-CSF),14 electroacupuncture,15 and regenerative medicine.16 However, some women with thin endometrium remain non-responsive even when these treatments have been performed.
Recently, autologous platelet-rich plasma (PRP) has been used in many medical fields such as trauma, neurology, osteoarthritis, aesthetics, orthopedics, ophthalmology, and wound healing.17,18,19,20,21,22,23 The composition of PRP contains many growth factors, cytokines, and proteins. In obstetrics, specifically for the treatment of the thin endometrium has not been widely applied in Vietnam.
This study was conducted to evaluate the effectiveness of autologous PRP on its own in treating patients with thin endometrium.
METHODS
A case series study was performed on 34 patients who agreed to participate and met the enrollment and exclusion criteria for autologous PRP treatment due to thin endometrium, from December 2020 to March 2021 in the Military Institute of Clinical Embryology and Histology. The patients were treated with hormone replacement therapy (HRT) in the previous cycles, but the endometrial thickness was < 7 mm. Their FET cycles were canceled at least twice.
In addition to HRT, autologous PRP was prepared from whole blood via 2 rounds of centrifugation and infused before the embryo transfer.
Selection criteria: endometrium thickness < 7 mm, with at least 2 good-quality embryos, at least 2 canceled FET due to thin endometrium in previous cycles.
Exclusion criteria: hydrosalpinx, fluid in the uterine cavity after c-section, fibroids, congenital uterine abnormalities.
Techniques
Endometrial preparation: using hormone replacement therapy with oral estradiol 8 - 12 mg from the second or third day of the cycle (Progynova®; Bayer AG, Germany).
Intrauterine infusion of autologous PRP was performed twice; the first time was on the 9th day of the cycle, and the second time was on the 12th or 13th day of the cycle. The endometrial thickness was evaluated by vaginal ultrasound after 2 days of PRP infusion. If the endometrial thickness was ≥ 7 mm after the second time of PRP infusion, it was transformed by vaginal micronized progesterone supplementation (Utrogestan®, Besins Healthcare, Drosgenbos, Belgium), 400 mg twice daily and oral dydrogesterone 20 mg (Duphaston®, Abbott healthcare product, the Netherland), which were commenced 3 or 5 days before transfer, depending on the day of embryo freezing. If serum level of Human chorionic gonadotropin (hCG) was above 25 mIU/mL on day 14 post-FET, progesterone was maintained for up to 12 weeks.
PRP separation technique: according to the guidance of Genenew World - Vietnam, 8 mL was taken of the patient's peripheral venous blood into a tube with 2.5 mL of Citrate Acid anticoagulant and centrifuged immediately at 1 200 revolutions per minute, for 10 minutes in order to separate plasma. Then, the plasma was transferred into a new sterile tube and then centrifuged at 3 300 revolutions per minute for 5 minutes, discarding the supernatant layer, leaving 1.5 ml of plasma at the bottom of the tube. The plasma was transferred into the PRP tube and mixed lightly, waiting for 5 - 7 minutes. A volume of 0.5 mL PRP was selected and infused into the uterine cavity with an intra-uterine insemination catheter (Gynetics®, Lommel - Belgium).
Variables
Number of FET cycles canceled due to the thin endometrial thickness.
Endometrial thickness before and after autologous PRP treatment.
Implantation rates.
hCG positive pregnancy rates (serum hCG level was above 25 mIU/mL on day 14 post-FET).
Clinical pregnancy rates.
Data analysis
The statistical analysis was performed using IBM SPSS software, version 16. Paired -Student t-test for the comparison of the differences between the pre-PRP and post-PRP endometrium thickness and mean + SD. A p value of < 0.05 was considered statistically significant.
Ethics Approval
This study was approved by the medical ethics committee of Vietnam Military Medicine University. All of the patients signed the written informed consent.
RESULTS
Thirty-four patients were treated with autologous PRP; on 6 patients the embryo transfer was due to endometrium thickness < 7 mm; 28 patients got the endometrial thickness ≥ 7 mm, and performed FET.
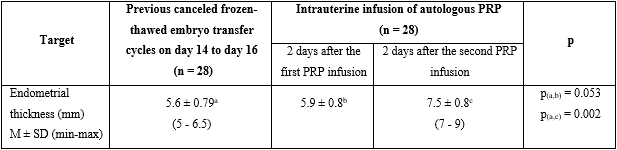
Table 1 presents that endometrial thickness of previous cycles was 5.6 ± 0.79 mm, embryo transferred cycles canceled due to thin endometrium were 3.43 ± 0.63. There were 23 patients with a history of abortion due to stillbirth and 5 patients with unknown causes.
Table 1 Characteristics of patients before autologous PRP treatment
| Characteristics | Patients with thin endometrium (n = 28) |
|---|---|
| Age | 32.93 ± 4.86 |
| Infertility years | 6.3 ± 3.7 |
| Endometrial thickness (mm) | 5.6 ± 0.79 (min: 5; max: 6,5) |
| The number of canceled embryos transferred cycles | 3.43 ± 0.63 (min: 3; max: 5) |
| 3 cycles canceled = 18 cases | |
| 4 cycles canceled = 8 cases | |
| 5 cycles canceled= 2 cases | |
| Causes | 23 cases have an abortion history (82.14 %) |
| 5 cases with unknown causes (17.85 %) |
Table 2 shows that the endometrial thickness was 5.9 ± 0.8 mm after the first time PRP infusion, which was not different from the previous cycles, with p= 0.053. On the second PRP infusion, the endometrial thickness was significantly improved by 7.5 ± 0.8 (min: 7 mm, max: 9 mm), which was different from the previous cycles with p= 0.002.
Table 2 The endometrial thickness after autologous intrauterine PRP infusion in patients with thin endometrium
Twenty-eight patients with endometrial thickness reached ≥ 7mm, and transfer frozen embryos was indicated. After 14 days, 14 patients had a pregnancy with rate 14/28 (50 %), the clinical pregnancy rate was 12/28 (42.85 %) and implantation rate was 23.07 % (table 3).
DISCUSSION
Kasius et al.5 reported an incidence of 2.4 % in their meta-analysis that included 1 170 patients undergoing in vitro fertilization.5 Most thin endometrial conditions were divided into primary and secondary causes. The primary cause group is due to the structure of the individual uterine. The secondary causes group includes acute and chronic inflammation; repeated abortion can lead to the damage of the basal layer of the endometrium. Hysteroscopic myomectomy, polypectomy or laparoscopic myomectomy may lead to intrauterine adhesions. Using clomiphene citrate for ovarian stimulation also leads to a thin endometrium.24
Recently, autologous PRP intrauterine infusion is a new therapy in order to support thin endometrium. The platelet concentration in PRP was 4 - 5 times higher than in peripheral blood.25 The platelets contain several growth factors and cytokines, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF) , epidermal growth factor (EGF) , fibroblast growth factor (FGF), insulin-like growth factor (IGF) I, II, interleukin 8 (IL-8) and connective tissue growth factor (CTGF). When tissue damage occurs, the cells that reach the site of injury are platelets. Anuclear platelets contain several types of granules involved in coagulation, inflammation, atherosclerosis, antimicrobial host defense, and angiogenesis. These a-granules contain many growth factors, including PDGF, TGF-β, VEGF, IGF, FGF, EGF, and keratinocyte growth factor, as well as many cytokines, chemokines, and resulting metabolites. PRP contains high levels of these autologous growth factors.26 For the first time, Chang et al.27 reported the efficacy of intrauterine infusion of PRP in 5 cases with thin endometrium in the previous cycles, who had poor response to conventional therapy in FET cycles. The results were improved endometrial thickness, 5 women got clinical pregnancy and 4 women got ongoing pregnancy.27
In 2019, Kim H et al.28 performed a comparison between previous cycles and post cycles with the treatment of PRP for 20 women with thin endometrium. As a result, the endometrium was 5.4 ± 0.8 mm in cases without the PRP therapy; in the PRP therapy group, the endometrium was 6.0 ± 1.1 mm, an increase of more than 0.6 mm, although the difference is not significant with p= 0.07. In the previous cycles without PRP treatment, including 20 cases (10 fresh embryo transfers and 10 frozen embryo transfers) there were no pregnancies. However, in the post cycles with PRP treatment, there were 6 cases of pregnancy. In the present study, patients with endometrial thickness ≥ 7mm after PRP treatment was indicated for frozen embryo transfer. Consequently, the endometrial thickness was thicker than in previous cycles without autologous PRP treatment, respectively (7.5 ± 0.8 mm and 5.6 ± 0.79 mm), and the clinical pregnancy rate was 12/28 (42.85 %). The procedure of autologous PRP preparation was safe and easy to follow. In addition, it avoids infected diseases and immune responses because the autologous PRP was derived from autologous blood.
Autologous platelet-rich plasma improves the endometrial thickness and the pregnancy rate outcomes in women with thin endometrium.















