Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Salud Animal
versión impresa ISSN 0253-570X
Rev Salud Anim. v.31 n.1 La Habana ene.-abr. 2009
Artículo original
SAFETY OFPochonia chlamydosporia var. catenulata IN QUAILS
SEGURIDAD DE Pochonia chlamydosporia var. catenulata EN CODORNICES
Liseth García, C. Bulnes, G. Melchor, L. Hidalgo, J. Figueredo, E. Vega y E. Marrero
Centro Nacional de Sanidad Agropecuaria (CENSA), Apartado. 10, San José de las Lajas, La Habana, Cuba. E-mail: lgarcia@censa.edu.cu
ABSTRACT
To obtain safe quality criteria for biological control agents, many tests have been developed. The aim of these studies was to determine the potentiality of Pochonia chlamydosporia var. catenulata to produce toxicity, infectivity or other organ/tissue damage in quails. Acute and toxicity/pathogenicity studies were performed by oral route. A maximum dose level of 6x106 units of the microbial pest control agent per bird treated was used. Mortality or clinical signs were not observed and no effects on body weight, haematology, microbiology and gross or microscopic pathology were detected. Thus clinical, microbiological and pathological results provided evidence for the safety properties of this fungus in quails.
Key words: Pochonia chlamydosporia var. catenulata; toxicity/pathogenicity; safety; quails
RESUMEN
Para obtener los criterios de calidad y seguridad de agentes de control biológico, muchas pruebas han sido desarrolladas. El objetivo de estos estudios fue determinar el potencial de Pochonia chlamydosporia var. catenulata para producir toxicidad / infectivity u otro daño en órganos o tejidos de codornices. Se realizaron estudios agudos y de toxicidad / patogenicidad por vía oral. Se evaluaron dosis máximas de 6x106 unidades del agente microbiano para el control de plagas. No se observaron signos clínicos, mortalidad ni ningún efecto sobre el peso corporal, la hematología, la microbiología y la patología macroscópica o microscópica. Por lo tanto, los resultados clínicos, microbiológicos y patológicos proveen evidencias de las propiedades de seguridad de este hongo en las codornices.
Palabras clave: Pochonia chlamydosporia var. catenulata; toxicidad/patogenicidad; seguridad; codornices
INTRODUCTION
The nematophagous fungus, Pochonia chlamydosporia var. catenulata (Kamyschlco ex Barron & Onions) Zare & Games (1), has been researched as a potential biocontrol agent for being used in integrated pest management strategy for Meloidogyne incognita (Kofoid and White) Chitwood in vegetable crops in Cuba by means of its application directly in the soil (2, 3).
To obtain safety aspects for biological control agents regarding registration, many tests of direct toxicity to beneficial species are required by the regulatory authorities around the world (4). Effects on terrestrial vertebrates exposed to contaminated food, soil, air, water or surfaces, have obvious economic and/or social consequences. Poisoned birds and mammals probably constitute the highest social concern and represent a high ecological hazard.
Many previous workers have studied various aspects of the toxicity and ecotoxicity of this strain including vertebrate tests with rats and rabbits (5, 6) and invertebrates using earthworms, bees, predaceous insects and non-target beneficial plants (7). No toxic, irritant, pathogenic and infective effects were detected; safety has been suggested in the use of this fungus.
In this study, quail was used as model; joined with the evaluations mentioned before, to provide a battery of test for assessing the ecotoxicological impact of IMI SD 187 of Pochonia chlamydosporia var. catenulata on non-target organisms.
MATERIALS AND METHODS
Test Material
In the studies, the fungus Pochonia chlamydosporia var. catenulata, IMI SD 187 was supplied by the Research and Development Unit of Biological Control for Agriculture, at CENSA.
The substrate colonized with the fungus and powders of pure chlamydospores of the fungus were the inocula used in the studies. To adjust chlamydospore concentration in the substrate colonized, 9mL of sterile distilled water were added to 1g of the substrate, the mixture became agitated vigorously and then proceeded to the count in a hematocytometer. For the variant of pure chlamydospores (PA), they were extracted from the substrate colonized after 21 days of incubation at 25°C, using a MycoHarvest, model Aeromatic-Fielder AG (CABI, Bioscience-Biopesticides Programme) and their concentration was determined in an equal form that the previous inoculum.
Test Animals
Young adult male and female Coturnix coturnix japonic quails, from 7 to 14 w-old were purchased from the Wajay Poultry Farm "26 of Julio", belonging to Poultry Genetic Company of Havana. They were housed in isolated area (Heppa filter, 99.9%) under standard 12 h light-dark cycles, a relative humidity of 50-70% and a temperature of 20±2°C and allowed freely to drink water and feed (sterilized by autoclaving). Previously to the experiment, a pre-acceptation study was carried out. All the studies were conducted according to the ethical principles for animal care and management recommended by the Good Laboratory Practice and Guidelines (8, 9).
Study Designs
In the acute oral study (EPA, OPPTS 885.4050), animals were allotted randomly into 2 groups. Group 1 (5 animals of each sex) received 0.5ml of sterile distilled water and served as control; and group 2 (5 animals of each sex) received 6 x 106 chlamydospores /animal suspended in 0.5mL of sterile distilled water (10). During the 2-w experimental period, animals were inspected daily for signs of toxic effects. They were weighed the day before dosing started and on days 3, 7 and 14. At the end of the study, animals were sacrificed for gross pathology.
For the toxicity/ infectivity/ pathogenicity study (EPA, OPPTS 885.4050), quails were treated during five days. Group 1: negative control (C), group 2 contact control (CC), group 3 treated (5 animals/sex) with 0.5mL of the inactive suspension of chlamydospores (ISC) and group 4 (25 animals/ sex) with 6x106 chlamydospores / animal of the suspension of chlamydospores (SC), were used. During the experimental period, animals were inspected daily for signs of toxic effects. They were weighed the day before dosing started and on days 3, 7, 14, 21 and 30. From group 4, on study days 3, 7, 14 and 30; 3 animals per sex, were sacrificed and blood was collected for haematology. The remaining animals from the control and dosage group were sacrificed at the end of the study and they all were subjected to a complete examination for any sign of gross pathology. Liver, kidneys, heart, spleen, lungs, brain, stomach, jejunum and any grossly observed lesions, were processed for histological examinations and for microbiological determinations. Blood samples were taken from the heart. Heparinized tubes were used for haemoglobin and haematocrit determinations. The fungus clearance was estimated examining faeces samples at 1, 3, 7, 24, 48, and 72 hours after treatment. 1g of faecal material per animal was triturated with 10 ml of physiologic saline solution (0.9%) and tested for microbial pest control agent clearance. Recovery values and detection of the fungi were determined by Standard Dilution Plating Technique (11). The total count was carried out in camera of Neubauer. To detect Pochonia chlamydospores in organs, liver, kidneys, heart, spleen, lungs, brain, stomach, jejunum from six rats, sacrificed at 3, 7, 14, 21 and 30 days post-treatment, were studied. A piece of each organ under study was put in culture medium on petri dish (Malt Extract Agar) with chloranfenicol, 25% and blood agar with chloranfenicol, incubated at 25ºC for 10 days. Samples were examined under light microscope (Zeizz-Axiolab, Magnification 40x) to detect the presence of the fungus.
In the acute study, data were analysed by Kruskall Wallis test. Wilcoxon test was used in the infectivity assay. A confidence limit of 95% was used as the critical significance level for all the tests.
RESULTS
Acute oral toxicity
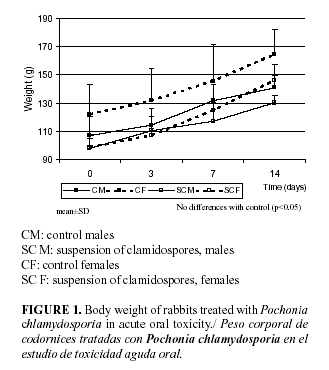
No death occurred during the test and there were neither treatment- related clinical signs, which confirms the normal behaviour in absolute body weights related with the controls (Fig. 1). No macroscopic alterations were observed in the organs.
Oral toxicity/ infectivity/pathogenicity
There were no treatment-related clinical signs of toxicity. When comparing the weight of the treated group with regard to the control, there was not any significant difference (p<0.05) in males; otherwise females showed differences (p<0.05) in the weight of the day 3 and 7. Although when analyzing the gain 0-3 and 3-7, there are no differences related to the control gain in the same period (Figure 2).
In the second day, one male of the control group appeared dead, without any apparent alteration; this is considered normal since it is below the limit used as approach of acceptance of the test, where more than 20% of the controls cannot die (10). Two males treated with the fungus showed a transitory breathing difficulty, after the administration of the product and they died for bronchoaspiration after the second and third treatment.
In the haematic analysis of quails (Table 1), the values of the red series were inside the physiologic range for the species. In the white series, an increment of the total leukocytes was observed, in days three and seven with regard to the controls, there were variations inside the physiologic range (12). This increment of the total leukocytes was related with the increase of the heterofiles and lymphocytes, with a later recovery of the first ones, starting from the 14 days post-treatment. Those two parameters showed values above the physiologic range; however they were not different to the control. The behaviour of the heterofiles on day 3 is directly associated with the process of bronchoaspiration of the two died animals. Day 7 could be related with the effect of the application of the inoculum, considering that the animals exposed were able to counteract the discreet bronchoaspiration with defensive mechanisms. The increase of lymphocytes could appear as a beneficial response of the organism to this agent (13).
In the microbiologic analysis of the faecal hexes in the samples, the presence of any microorganism of interest was not detected, neither the presence of chlamydospores of the fungus; it means that after being administered during five serial days, the fungus was eliminated during the first 48 hours post-treatment (Table 2). No growth of the microorganism evaluated in none of the organs of the animals examined was observed.
From the macroscopic point of view, the presence of a discreet congestion in several organs of all groups including control was observed as element of interest. It was in correspondence with the results of the histopathology analysis. No alterations in the relative weight of the organs were observed (Table 3).
DISCUSSION
A finding of acceptable risk does not mean that under every circumstance a product will never prove harmful. Burges (14) stated that "Registration of a chemical is essentially a statement of usage in which the risks are acceptable. The same must be applied to biological agents". Even when products have successfully cleared these hurdles, new questions can arise based on the changing public perception of risk.
From the clinical point of view, a toxic influence is not detected on the treatment since in animals, there were not alterations in evolution of weight, or clinical signs of toxicity, and the haematic values as well as the behaviour of the animals were normal during rehearsals.
Likewise, there was not any infection evidence neither multiplication indications, what is attributed to those chlamydospores which did not germinate, that is they behaved as inert particles, with a quick clearing where the number of recovered organisms diminishes quickly in the time (15).
In general, the pathological changes were not consistently associated in particular with any group of treatment, what could be associated to the low capacity of being distributed and to persist in the body of the animals treated.
These results are similar to the findings of Vestergard et al., 2003 (16) when evaluating the fungus M. anisopliae in birds feeding them with contaminated insects with their conidia; and no toxic effect on the behaviour, neither on other clinical variables analyzed during the tests were obtained.
CONCLUSION
The strain IMI SD 187 of Pochonia chlamydosporia var. catenulata does not turn out to be toxic, infective, or pathogenic for quails.
REFERENCES
1. De Leij FAMM and Kerry BR. The enmatophagus fungus Verticillium chlamydosporium as a potential biological control agent for Meloidogyne arenaria. Revue de Nematologia. 1991;14:157-164.
2. De Leij FAMM, Kerry, BR and Dennehy JA: Verticillium chlamydosporium as a biological control agent for Meloidogyne incognita and M. hapla in pot and microplot test. Nematologia. 1993;39:115-126.
3. Hidalgo-Diaz L, Bourne JM Kerry BR and Rodriguez MG: Nematophagous Verticillium spp. in soils infested with Meloidogyne spp. in Cuba: isolation and screening. International Journal of Pest Management. 2000;46:277-284.
4. Goettel MS, Jaronski ST: Safety and registration of microbial agents for control of grasshoppers and locusts. Memoirs of the Entomological Society of Canada. 1997;171:83-99.
5. García L, Bulnes C, Melchor G, Vega E, Montes de Oca N, Hidalgo L y Marrero E: Safety of Pochonia chlamydosporia var. catenulata on acute oral and thermal toxicity/pathogenicity evaluations in rats and rabbits. Vet Hum Toxicol. 2004;46(5):248-250.
6. García L, Melchor G, Montes de Oca N, Hidalgo L: Estudio de la irritación ocular y dérmica de Pochonia chlamydosporia var. catenulata. Rev. Tox. 2004;21(2-3):103-107.
7. García L, Melchor G, Arévalos G y Hidalgo L: Evaluación de la fitotoxicidad de la cepa IMI SD 187 Pochonia chlamydosporia var. catenulata sobre Zea mays L. y Phaseolus vulgaris L. Rev. Sanidad Vegetal. 2007;23:2.
8. Landsdown AB: Animal husbandry. In: Anderson D (Ed), Experimental Toxicology, The basic issues. BIBRA, Surrey, UK, p. 101, 1993.
9. Ewbank R, Godfrey C, Holgate B, Inglis I, James R, et al.: Refining procedures for the administration of substances. Lab Anim. 2001;35:1-41.
10.Environmental Protection Agency (EPA): Prevention, Pesticides and Toxic Substances. Microbial Pesticides Test Guidelines. OPPTS 885.4050, Acute Oral Toxicity/Pathogenicity, 1996.
11.Kerry BR, Kirkwood IA, De Leij FAAM, Barba J, Leidjens MB, Brookes PC: Growth and survival of Verticillium chlamydosporium Goddard, a parasite of nematodes in soil. Biocontrol Science and Technology. 1993;3:355-365.
12.Olfert DE, Brenda M, Ann M: Guide to the care and use of experimental animals. 2nd ed. Appendix IV; 1993;1:174.
13.Swenson MJ: Physiological Properties and Cellular and Chemical Constituents of Blood. Chapter 2. En: Swenson MJ. Tenth eds. Dukes´ Physiology of Domestic Animals. Comstock Publishing Associates, Cornell University Press. Ithaca and London, 1984.
14.Burges HD (ed.): Microbial Control of Pests and Plant Diseases 1970-80. New York: Academic Press, 1981.
15.Siegel Siegel JP, Shadduck JA: Some observations on the safety of the entomopathogen to mammals. Journal of Economic Entomology. 1987;80:994-997.
16.Vestergard S, Cherry A, Keller S, Goettel M: Safety of hyphomycete fungi as microbial control agents. En: Hokkanen HMT, Hajek AE (eds). Environmental Impacts of Microbial Insecticides. Kluwer Academic Publishers. Netherlands, 35-62, 2003.
(Recibido 24-4-2008; Aceptado 15-7-2008)














