Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de Salud Animal
versión impresa ISSN 0253-570Xversión On-line ISSN 2224-4700
Rev Salud Anim. vol.40 no.1 La Habana ene.-abr. 2018
ARTÍCULO ORIGINAL
In vitro antiviral activity of Rhizophora mangle L. aqueous bark extract and the butanolic fraction against Canine Distemper Virus and Bovine Herpes Virus type 1
Actividad antiviral in vitro del extracto acuoso de corteza de Rhizophora mangle L. y la fracción butanólica contra el virus del Moquillo Canino y el virus del Herpes Bovino Tipo 1
Elizabeth de Armas1, Alessandra Scagliarini2, Mara Battilani2, Pastor Alfonso1, Evangelina Marrero1*
1Animal Health Direction, National Centre for Animal and Plant Health (CENSA), San José de las Lajas, Apdo. 10, CP. 32700, San José de las Lajas, Mayabeque, Cuba.
2Department of Veterinary Medical Science, Alma Mater Studiorum, University of Bologna, Italy.
ABSTRACT
There is currently an increasing need for new substances or secondary metabolites from natural products with antiviral activity. The aim of this study was to determine the antiviral activity of Rhizophora mangle L. against DNA and RNA virus. With this aim, R. mangle aqueous bark extract (RMABE) and its butanolic fraction (BF-RMABE) were evaluated for the cytotoxicity and cytopathic effect inhibition assay. The selectivity index was also calculated. An in vitro antiviral activity was demonstrated for RMABE and BF-RMABE against the RNA prototype of Canine Distemper Virus (CDV) (CI50 83.30 and 86.10 μg/mL, respectively) and the DNA prototype of Bovine Herpes Virus type 1 (BHV-1) (CI50 87.50 and 90.63 μg/mL, respectively). The results showed the promising antiviral effect of the evaluated R. mangle extracts, suggesting the need for further tests to confirm itsin vivo activity.
Key words:in vitro antiviral activity, CDV, VHB-1, polyphenols, Rhizophora mangle.
RESUMEN
Actualmente existe una creciente necesidad de nuevas sustancias o metabolitos secundarios a partir de productos naturales con actividad antiviral. El objetivo de este estudio fue determinar la actividad antiviral de Rhizophora mangle L. frente a virus prototipos de ADN y ARN. En este sentido, se evaluó el extracto acuoso de corteza de R. mangle (RMABE) y su fracción butanólica (BF-RMABE) mediante las pruebas de citotoxicidad e inhibición del efecto citopático. De la relación entre estas pruebas se calculó el índice de selectividad. Se demostró, además, actividad antiviral in vitro para RMABE y BF-RMABE frente al virus del moquillo canino (CDV) como prototipo de virus ARN (CI50 83,30 y 86,10 μg/mL, respectivamente) y el Herpes Virus Bovino tipo 1 (BHV-1) como prototipo de ADN (CI50 87,50 y 90,63 μg/mL, respectivamente). Los extractos de R. mangle evaluados, mostraron efecto antiviral, lo que sugiere la necesidad de pruebas adicionales para confirmar su actividad in vivo.
Palabras clave: actividad antiviral in vitro, CDV, BHV-1, polifenoles, Rhizophora mangle.
INTRODUCTION
Plants are a natural source of therapeutically recognized compounds with diverse biological activities offering treatment for several human and animal diseases. Viral infections represent an important research focus for the identification of new phytopharmaceuticals (1).
The discovery of antivirals is a relatively recent issue. At present, the antiviral therapy is mostly limited to human diseases with few applications in veterinary medicine. Currently the research carried out on antivirals against animal viruses is mostly aimed at providing new insights for the development of chemotherapeutics against phylogenetically correlated human viruses. There is in fact an increasing interest to the animals and their pathologies as possible models for human diseases. In the veterinary field, antivirals may represent an alternative when vaccination is not feasible in case of sudden epidemic outbreaks and they would offer advantages when in-contact animals need protection during emergency vaccinations. Although the primary focus has been on the synthetic products, the number of natural compounds with antiviral action for different DNA and RNA viruses that are being studied is increasing (2).
Tannins are secondary metabolites characterized by their polyphenol nature and they can be found in many plants as individual components or forming complex mixtures (3). There is a wide range of the pharmacological activity of the plants containing polyphenols as reported by Keerthi et al. (4). The wide biological activities of polyphenols have been related to their ability to bind macromolecules such as proteins and polysaccharides (5,6,7).
The antioxidant and radical scavenging activities of tannins are often linked to the interaction with metal ions, forming chelated complexes (8). The antiviral activity of the phenolic compounds against several DNA and/or RNA viruses has been well documented by Chattopadhay et al. (9), Bhanuprakash et al. (10), Elsebai et al. (11). Thus, Gamaleldin et al. (12), reported that plants, rich in chlorogenic acids (CGAs), caffeic acids and their derivatives, have been found to exert the antiviral effects against influenza virus neuraminidase. The crude and aqueous extract and the ethyl acetate fractions of Trichilia catigua (a Brazilian native plant) have been reported to exert the antiviral activity during the replication of the Herpes Simplex Virus (HSV-1), BHV-1 and Poliovirus (PV-1), which are responsible for important diseases in humans and animals (13).
The phenolic compound eugeniin (ellagitannin), extracted from Geum japonicum and Syzygiuma romaticum, clearly demonstrated the anti-HSV activity by decreasing the DNA polymerase activity (14).
In a systematic study of the antiviral activity of a very wide range of natural products, Van den Berghe et al. (15) concluded that polyphenols act principally by binding the virus and/or the protein of the host cell membrane being able to inhibit the absorption of several viruses. Further researches demonstrated the capability of polyphenols to inactivate proteins from the cell surface, to block the viral adsorption and to inhibit the viral enzymes such as reverse transcriptase and RNA polymeraseand poly (ADP-ribose) (16).
Steinmann et al. (17) reported the antiviral activity of epigallocatechin-3-gallate (EGCG), the main constituent of Camellia sinensis (green tea), showing different mechanisms of action on diverse families of viruses, such as Retroviridae, Orthomyxoviridae and Flaviviridae, as well as important human pathogens such as the human immunodeficiency, influenza A and hepatitis C viruses. Furthermore, the molecule demonstrated to be able to interfere with the replication cycle of DNA viruses like hepatitis B virus, herpes simplex virus and adenovirus.
Another example of the antiviral effect of polyphenols is that of bakuchiola phenolic isoprenoid with novel enantiomer-selective anti-influenza A virus activity involving Nrf2 activation. Compounds like (+)-(S)-bakuchiol and its enantiomer, (-)-(R)-bakuchiol, inhibit influenza A viral infection and growth and reduce the expression of viral mRNAs and proteins in these cells. Furthermore, these compounds markedly reduced the mRNA expression of the host cell influenza A virus-induced immune response genes, interferon-β and myxovirus-resistant protein 1. Interestingly, (+)-(S)-bakuchiol had a greater efficacy than (-)-(R)-bakuchiol, indicating that chirality influenced the anti-influenza virus activity (18).
Rhizophora mangle L, red mangrove, is recognized in the Cuban traditional medicine and in different Caribbean countries as well (19). Its aqueous bark extract showed to be rich in polyphenols (20). R. mangle revealed interesting pharmacological effects with therapeutic potentiality such as: antimicrobial (21), wound healing promoter (22), anti-inflammatory (23), in the treatment of aphthous ulcer (24), antioxidant (25), and antiulcerogenic (26). The chemical composition of Rhizophora mangle aqueous bark extract and its fractions were also studied in previous researches (26,27).
It is worth mentioning that polyphenols may modulate the production of IL-2 and interferon gamma (IFN-gamma). Thus following that logic, it was confirmed that the structural diversity of polyphenols present in Rhizophora mangle L has a capacity to stimulate the release of IL-2, indicating that such polyphenols play an important role in the biological regulations (27).
Orf, commonly referred as contagious ecthyma, is a highly contagious viral disease that causes important economic losses in sheep and goats, being one of the most skin distressing diseases affecting these species. The topical application of Rhizophora mangle aqueous bark extract on lambs experimentally infected with Orf virus shows a faster healing than in the treated group (28).
The current work researched the in vitro antiviral activity of Rhizophora mangle aqueous bark extract (RMABE) and its butanolic fraction (BF-RMABE) against BHV-1 and CDV as prototypes of DNA and RNA viruses respectively.
MATERIALS AND METHODS
Rhizophora mangle aqueous bark extract (RMABE) and its butanolic fraction (BF-RMABE)
Fresh bark in distilled water (1:7.5 w/v) was boiled for 20 min. The plant material was separated by filtration and the supernatant (RMABE) was freeze-dried and preserved at 4-8°C.
BF-RMABE fractionation was carried out with n-butanol as follow: 50 mL of distilled water and 18 g NaCl were added to 1g of RMABE. The solution was stirred, heated (78°C) for 30 minutes and centrifuged at 3000 rpm for 10 minutes. The precipitate was then extracted with n-butanol using Büchi B 811 Extraction System (upper heating 4, lower heating 16, 2:00 hours) and dried by rotary evaporation (55°C).
Cells and viruses
VERO cells (culture collection ATCC® number CCL-81™) and MDBK cells (culture collection ATCC® number CCL-22) were used for the in vitro growth of canine distemper virus (CDV) and bovine herpes virus- 1 (BHV-1) respectively. Cells were cultured at 37 °C in a 5 % CO2 atmosphere in Dulbecco’s modified Eagle’s medium (Gibco, Invitrogen Corporation) supplemented with 10 % fetal calf serum (FCS), 2 mmol/L of L-glutamine, 1 mmol/L of sodium pyruvate and 7.5 % sodium bicarbonate. All the cells, medium components and virus strains were previously tested for the absence of Mycoplasma spp. and Bovine Viral Diarrhea virus (BVDV) by PCR assays. Bussell-CDV strain and BoHV-1 have been propagated in cells using a 2 % FCS medium and they were subsequently titrated. The viral titer was expressed as the 50 % tissue culture infectious dose (TCID50/mL).
Cytotoxicity assay
The cell toxicity of RMABE, BF-RMABE, ribavirin (RBV) and HPMPC (S-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine was evaluated on the growing cell lines VERO and MDBK using a colorimetric assay based on the mitochondria metabolization of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, Missouri). The toxicity of the compounds and DMSO used to dissolve them was measured as previously described by Scagliarini et al. (29). The 50 % cytotoxic concentration (CC50) was defined as the compound concentration able to reduce the number of viable cells to 50 % of cell control. The CC50 values were expressed as the mean ± the standard deviation of at least three independent experiments.
Antiviral assays
The intracellular activity of the plant extracts and positive controls 1-β-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide (Ribavirin, RBV) and (S)-1-(3-Hydroxy-2-phosphonylmethoxypropyl) cytosine (HPMPC, Cidofovir, Vistide) against animal viruses was evaluated by the cytopathic effect (CPE) reduction assay (CPE-reduction assay) on confluent cells, using the protocol previously described by Scagliarini et al. (29).
Statistical analysis
The selectivity index (SI) was obtained by calculating the calculating the ratio between the cytotoxicity and the antiviral activity values.
The 50 % inhibitory concentration (IC50), defined as the compound concentration required to reduce viral CPE by 50 % of the virus control, was calculated as the mean of three independent experiments ± the standard deviation.
RESULTS AND DISCUSSION
The cytotoxicity assays allowed determining the cytotoxic concentration (CC50) of RMABE and BF-RMABE in Vero and MDBK cells (Table 1). The cytotoxicity assessment is clearly an important part of a potential antiviral agent evaluation because a useful compound should be selective for virus-specific processes with no or few effects on cellular metabolism and not showing toxicity against the host (30,31).Additionally, this step is critical because the virus uses the cell machinery to replicate, and it must be ensured that the virus has the ideal conditions for growth (32).
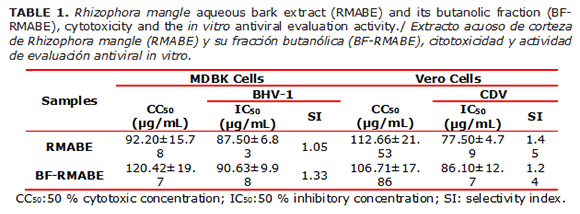
The present results showed relatively low CC50 values for RMABE and BF-RMABE in Vero and MDBK cells compared to the control compounds HPMPC and RBV. These results are in accordance with previous results obtained with Sesbania virgata extracts showing CC50 values between (3.91 and 250 μg/mL) and (15.62 y 125 μg/mL) for MDBK and Vero cells respectively. The polar nature of the active ingredient(s) in the antiviral activity of this plant was suggested (33).
Ruibal et al. (34) found high cytotoxicity for different polyphenolic fractions obtained from Pinus caribaea Morelet var. caribaea in MT4 cells. Fractions containing condensed tannins showed CC50 = 100 μg/mL; while low molecular weight phenolics were highly toxic to the cells. In this sense, it was previously demonstrated that the presence of hydroxyl groups is associated to the increment of intracellular reactive oxygen species (ROS), with consequences in the damage of the cells, although the relationship among compounds with similar structures and cytotoxicity is still unknown for particular types of cells (35).
Therefore, a Phyllanthus orbicularis extract was evaluated using MTT assay and cytotoxicity was determined for MDBK, FPH, HeLa and HEp-2 cells, showing CC50 values of 262.05±12.7, 669±36, 212.62±8.37 and 144.7±3.7 μg/mL, respectively (36).
However, compounds containing hydroxyl groups can interact with iron, copper and other metals usually present in culture media and fetal bovine sera, intensifying MTT reduction and increasing blue coloration. Some compounds can react with iron, generating blue-black complexes thatmay influence the cytotoxicity estimation through MTT assay (30).
The results obtained in the in vitro antiviral assay are shown in Table 1.
Thus, current results showed antiviral activity of RMABE and BF-RMABE against BHV-1 and CDV in MDBK and Vero cells, respectively. Nevertheless, these preliminary results did not show differences between both types of viruses and IC50 values were found between 83.30 and 90.63μg/mL. To screen the antiviral activity of plant extracts, it is important to choose animal viruses containing the main differentiating features of viral morphology, like viruses with no envelope, those with an envelope, as well RNA and DNA viruses (32). In the current work, a prototype DNA virus (BHV-1) and an RNA virus, both enveloped, were used. This caused severe animal diseases. Particularly, CDV which is a Morbillivirus, is identified as an attractive target in the development of antiviral molecules, because human and animal tissues lack of a known homologue of the RNA-dependent RNA polymerase (33).
The current experiment also suggested that the evaluated extracts could affect viruses of the same genus or families, as well viruses with same genetic material. In fact, the topical application of Rhizophora mangle aqueous bark extracts RMABE and BF-RMABE in the treatment of contagious ecthyma in lambs experimentally infected with Orf virus (Poxviridae family), shows efficacy at the initial state of the infection (28).
Chattopadhay et al. (9) well documented the antiviral activity of the plant phenolic compounds against several DNA and/or RNA viruses. Cellular surface proteins are inactivated by polyphenols; they also interfere on viral adsorption, inhibit reverse-transcriptase and viral RNA polymerase, as well as DNA and RNA replication (16) Besides, studies related to structures and the antiviral biological activity of plants revealed that the sites and numbers of hydroxyl groups of phenols are responsible for their antiviral activity (9,15).
Camellia sinensis (green tea), catechins, epigallocatechin gallate (EGCG) have received the most attention and they have been researched deeply in its antibacterial, antiviral, and antifungal activities. EGCG was found to prevent influenza virus infections by binding the viral hemagglutinin, thereby preventing the attachment of viral particles to the target receptor cells. The antiviral action mechanisms of EGCG vary depending on its target virus (38).
A valuable review about the antimicrobial effect of polyphenols including the antiviral effect, by Daglia (39), mentioned that the activity can be attributable to both direct actions against bacteria, virus and fungi, as well as to the suppression of microbial virulence factors. Rhizophora mangle L shows ability to stimulate the in vitro release of IL-2, and such results could indicate that the polyphenols present in the plant play an important role as immune modulator as well (27). Gallina et al. (40) report that proanthocyanidin A2 extracted from Heasculus hyppocastanum exerted in vitro antiviral activity against CDV with a higher SI compared to Ribavirin. The in vitro antiviral activity of chestnut and quebracho wood extracts against avian reovirus and metapneumovirus has also been demonstrated by Lupini et al. (41).
Thus, Ikuno et al. (34) evaluated Sesbania virgata leaf extracts against BHV-1 and HVS-1 in MDBK and Vero cells. The results showed that all the fractions evaluated had a promising activity in MDBK cells against BHV-1. On the contrary, just two fractions (the most polar) showed moderated active in Vero cells against both BHV-1 and SHV-1 viruses. Different cell substrates were used for each virus. In this sense, the obtained results are not conclusive.
Although the massive use of antivirals in animals may be controversial over possible resistance, there are several animal diseases caused by DNA viruses like Orf and BHV-1 among others, which induce skin or mucosal erosive lesions needed to be treated with wound healing compounds. In such cases, RMABE and BF-RMABE, according to their healing properties (22), would be valuable therapeutic options contributing to animal welfare during infections. This is in agreement with the efficacy observed for these compounds in lambs experimental infected with contagious ecthyma (28). These viruses are quite stable reducing the probability of the emergence of resistant strains.
BHV-1 produces clinical manifestations characterized by pustular vulvo vaginitis and erosive skin lesions that may be treated with the evaluated compounds taking advantage of their antiviral effect combined with the wound healing properties (22). In fact, some other herperviruses also induce these kinds of erosive lesions that could be resolved by the potential similar mechanisms of antiviral effect.
Another factor influencing the antiviral activity is the virus strain. Thus, Savi et al. (30) reported different antiherpetic activities for epicatechin, epigaliocatechin and epigaliocatechin gallate against KOS and 29R/acyclovir resistant strains. Epicatechin showed the major activity.
Date and Destache (42) published data from various in vitro and in vivo studies carried out on epigallocatechin gallate, theaflavins (black tea polyphenols), resveratrol, genistein, and curcumin, highlighting their potential to prevent sexually transmitted infections caused by HIV (human immunodeficiency virus), HSV (herpes simplex virus) and HPV (human papilloma virus), confirming the potential of natural products as antiviral agents.
Moreover, the time and the moment for the plant extract sample application determine the intracellular or extracellular action. If the sample is not evaluated in an assay that is suitable for determining its mechanism of action, negative false results can be obtained and its antiviral potential could be limited. The present study evaluated RMABE and BF-RMABE against BHV-1 and CDV through an intracellular mechanism, inhibiting viral replication. This is an important issue because the severity of viral diseases and the ability of viruses to survive intracellularly pose a great challenge that is further aggravated by the non-availability of specific antiviral agents against veterinary pathogens (32). Other factors such as treatment time and infective viral doses could also influence the antiviral activity.
Besides, an intrinsic constituent of the antiviral testing is the determination of a selectivity index (SI) towards the supporting host cell. The SI refers to the ratio of the maximum drug concentration causing either 50 % or 90 % growth inhibition of normal cells (CC50, CC90) and the minimum drug concentration at which 50 % or 90 % of the virus is inhibited (IC50, IC90) (43).
The present results showed low SI values for RMABE and BF-RMABE compared to the chemical compounds tested for the antiviral activity against CDV (43). Ribavirin in Vero cells is active against CDV with a time-dependent antiviral activity. However, Ribavirin is approved for use in human, with an SI (1.2) in part justified due to its cytostatic activity (29). The antiviral and toxic effects are not necessarily caused by the same mix components in crude extracts; that is why SI is not as valued as when it is calculated for the pure or synthetic compounds.
1. Armendáriz-Barragán B, Zafar N, Badri W, Galindo-Rodríguez S, Kabbaj D, Fessi H, Elaissari A. Plant extracts: from encapsulation to application. Expert Opin Drug Deliv. 2016; May 17:1-11.
2. Garro HA1, Pungitore CR. Coumarins as Potential Inhibitors of DNA Polymerases and Reverse Transcriptases. Searching New Antiretroviral and Antitumoral Drugs. Curr Drug Discov Technol. 2015;12(2):66-79.
3. Ekambaram SP, Perumal SS, Balakrishnan A. Scope of Hydrolysable Tannins as Possible Antimicrobial Agent. Phytother Res. 2016 Jul; 30(7):1035-45. doi: 10.1002/ptr.5616. Epub 2016 Apr 7.
4. Keerthi M, Lashkmi PJ, Santhosh A M, Rama RM. Review on Polyphenols as nature gift. World Journal of Pharmacy and Pharmaceutical Sciences. Review article. 2014;3(4): 445-455.
5. Zhang YB, Wu P, Zhang XL, Xia C, Li GQ, Ye WC, Wang GC, Li YL. Phenolic Compounds from the Flowers of Bombax malabaricum and Their Antioxidant and Antiviral Activities. Molecules. 2015;20(11):19947-19957.
6. Al-Fatimi M, Ali NA, Kilian N, Franke K, Arnold N, Kuhnt C, Schmidt J, Lindequist U. Ethnobotany, chemical constituents and biological activities of the flowers of Hydnora abyssinica A.Br. (Hydnoraceae). Pharmazie. 2016;71(4):222-226.
7. El-Ashmawy IM, Al-Wabel NA, Bayad AE. Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pac J Trop Med. 2016;9(3):228-234.
8. Weidner S, Rybarczyk A, Karamac M, Król A, Mostek A, Grebosz J, Amarowicz R. Differences in the phenolic composition and antioxidant properties between Vitis coignetiae and Vitis vinifera seeds extracts. Molecules. 2013;18(3):3410-26.
9. Chattopadhay D, Arunachalam G, Mandal AB, Bhatachary SK. Dose dependent therapeutic anti-infectives from ethnomedicines of Bay Islands. Chemotherapy. 2006;52:151-157.
10. Bhanuprakash V, Hosamani M, Balamurugan V, Granhale P, Singh RK, Swarup D. In vitro Antiviral Activity of Eugenia Jambolana Plant Extract of Buffalopox virus: Conventional and q PCR Methods. Intern J Trop Med. 2007; 2(1):3-9.
11. Elsebai MF, Abass K, Hakkola J, Atawia AR, Farag MA. The wild Egyptian artichoke as a promising functional food for the treatment of hepatitis C virus as revealed via UPLC-MS and clinical trials. Food Funct. 2016;7(7):3006-3016.
12. Gamaleldin KM, Matei MF, Jaiswal R, Illenberger S, Kuhnert N. Neuraminidase inhibition of Dietary chlorogenic acids and derivatives - potential antivirals from dietary sources. Food Funct. 2016;7(4):2052-2059.
13. Espada SF, Faccin-Galhardi LC, Rincao VP, Bernardi AL, Lopes N, Longhini R, de Mello JC, Linhares RE, Nozawa. Antiviral Activity of Trichiliacatigua Bark Extracts for Herpesvirus and Poliovirus. Curr Pharm Biotechnol. 2015;16(8):724-732.
14. Kurokawa M, Hosumi T, Basnet P, Nakano M, Kadota S, Namba T, Kawana T, Shiraki K. Purification and characterization of eugenin as an antiherpesvirus compound from Geum japonicum and Syzygiuma romaticum. J. Pharmacol. Exp. Ther. 1998; 284(2):728-735.
15. Vanden Berghe DA, Vlietinck AJ, Vanhoof L. Plants products as potential antiviral agents. Bull Inst Pasteur. 1986; 84:101-147.
16. Jassim SA y Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95:412-427.
17. Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br J Pharmacol. 2013;168 (5):1059-73. doi: 10.1111/bph.12009.
18. Shoji M, Arakaki Y, Esumi T, Kohnomi S, Yamamoto C, Suzuki Y, Takahashi E, Konishi S, Kido H5, Kuzuhara T. Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation. J Biol Chem. 2015290(46):28001-28017.
19. Roig JT. Plantas medicinales, aromáticas o venenosas de Cuba. La Habana: Ed. Ciencia y Técnica. 1974.
20. Sánchez LM, Melchor G, Alvarez S, Bulnes C. Caracterización química y toxicológica de una formulación cicatrizante de Rhizophora mangle L. Rev. Salud Anim. 1998;20(2):69-72.
21. Melchor G, Armenteros M, Fernández O, Linares E, Fragas I. Antibacterial activity of Rhizophora mangle bark. Fitoterapia. 2001; 72:698-691.
22. Fernández O, Capdevila JZ, Dalla G, Melchor G. Efficacy of Rhizophora mangle aqueous bark extract in the healing of open surgical wounds. Fitoterapia. 2002;73:564-568.
23. Marrero E, Sánchez J, de Armas E, Escobar A, Melchor G, Abad MJ, et al. COX-2 and sPLA2 in vitro inhibitory activity from aqueous extract and high and low molecular weight polyphenols fractions obtained from Rhizophora mangle (red mangrove). Fitoterapia. 2005;77: 313-315.
24. de Armas E, Sarracent Y, Marrero E, Fernández O, Branford-White C. Efficacy of Rhizophora mangle queous bark extract (RMABE) in the treatment of aphthous ulcers: a pilot study. Curr Med Res Opin. 2005; 21(11):1711-1715.
25. Sánchez J, Melchor G, Martinez G, Escobar A, Faure R. Antioxidant activity of Rhizophora mangle bark. Fitoterapia. 2016; 77:141-143.
26. Perera LMS, Piloto J, Canelsota D, Pelzer L, Mancebo B. Further Pharmacological Evidence Supporting the Development of an Antiulcerogenic Drug Based on Rhizophora mangle L. Aqueous Extract. HPLC Method Proposed for Determinating a Chemical Marker. Open Access Library Journal. 2016;3:e1625http//dx.doi.org/10.4236/oalib.1101625.
27. de Armas E, Escobar A, Faure R, Marrero E, Bligh ASW, Branford-White CJ, White KN. Stimulation of Interleukin-2 [IL] Release by Rhizophora mangle Bark Aqueous Extracts and its Fractions. European Journal of Medicinal Plants. 2016;15(2):1-10.
28. de Armas Sanabria E, Oliva Hernández Y, Pérez Hernández Z, Ayala Galindo J, Martínez Marrero N, Fernández Limia O, Faure García R, Barreras M, Alfonso Zamora P, Marrero Faz E. Extracto acuoso de la corteza de Rhizophora mangle (RMABE) en un modelo de ectima contagioso en carneros infectados experimentalmente. REDVET Rev. Electrón Vet. 2012;13(6). http://www.veterinaria.org/revistas/redvet/n060612.html
29. Scaglarini A, Vaccari F, Gallina L, Dal Pozzo F, Prosperi S. In Vitro Evaluation of Antiviral activity of Ribavirin against Canine Distemper Virus. Vet Res Communicat. 2006;30(1): 269-272.
30. Savi AL, Barardi RC, Simoes MOC, Evaluation of antiherpetic activity and genotoxic effects of Tea Catechin derivatives. J Agric Food Chem. 2006;54(7):2552-2557.
31. Trejo-Avila LM, Morales-Martínez ME, Ricque-Marie D, Cruz-Suarez E, Zapata-Benavides P, Morán-Santibañez K, et al. In vitro anti-canine distemper virus activity of fucoidan extracted from the brown alga Cladosiphono kamuranus. Virus Dis. 2014;25(4):474-480. DOI 10.1007/s13337-014-0228-6.
32. Kohn LK, Queiroga CL, Martini MC, Barata LE, Porto PSS, Souza L, et al. In vitro antiviral activity of Brazilian plants (Maytenus ilicifolia and Aniba rosaeodora) against bovine herpesvirus type 5 and avian metapneumovirus. Pharmaceutical Biol. 2012;50(10): 1269-1275.
33. Bourhis JM, Canard B, Longhi S. Structural disorder within the replicative complex of measles virus: functional implications. Virol. 2006;344:94-110.
34. Ikuno A. Antiherpes activities of fractions from Sesbania virgate leave Arq. Inst. Biol. São Paulo. 2003;70(2):183-185.
35. Ruibal IJB, Dubed ME, Martínez FL, Noa ER, Vargas LMG, Santana JLR. Inhibición de la replicación del VIH por extractos de taninos de Pinus caribaea Morelet. Rev Cubana de Farmacia. 2003;37(2):1-7.
36. Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward culture normal human cells. Biol Pharm Bull. 2005;28:253-259.
37. del Barrio G. 1999. Actividad antiviral in vitro del extracto acuoso de Phyllanthus orbicularis, HBK. [Tesis presentada en opción al grado de Doctor en Ciencias Biológicas] Ciudad de la Habana.
38. Nakayama M, Suzuki K, Toda M, Okubo S, Hara Y, Shimamura T. Inhibition of infectivity of influenza virus by tea polyphenols. Antiviral Res 1993, 21:289-299.
39. Daglia M. Polyphenols as antimicrobial agents. Current Opinion in Biotechnology. 2012; 23:174-181.
40. Gallina L, Dal Pozzo F, Galligioni V, Bombardelli E, Scagliarini A. Inhibition of viral RNA synthesis in canine distemper virus infection by proanthocyanidin A2. Antiviral Research. 2011;92(3):447-452.
41. Lupini C, Cecchinato M, Scagliarini A, Graziani R, Catelli E. In vitro antiviral activity of chestnut and quebracho woods extracts against avian reovirus and metapneumovirus. Res Vet Sci. 2009;87(3):482-487.
42. Date AA, Destache CJ. Natural polyphenols: potential in the prevention of sexually transmitted viral infections. Drug Discov Today. 2016;21(2):333-341.
43. Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of products: How to develop a stronger in vitro prop-of-concept. J Ethnopharmacol. 2006;106:290-302.
44. Dal Pozzo F, Galligioni V, Vaccari F, Gallina L, Battilani M, Scagliarini A. Antiviral efficacy of EICAR against canine distemper virus (CDV) in vitro. Res Vet Sci. 2010;88:339-344.
Recibido: 10/11/2017
Aceptado: 10/1/2018
*Autor para correspondencia: Evangelina Marrero-Faz. E-mail: evangelinamarrerofaz@gmail.com














