My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Cultivos Tropicales
Print version ISSN 0258-5936On-line version ISSN 1819-4087
cultrop vol.40 no.2 La Habana Apr.-June 2019 Epub June 01, 2019
Original Article
Micropropagation and molecular characterization of a coffee variety (Coffea arabica) resistant to rust (Hemileia vastatrix)
1Universidad de Talca, Talca, Maule, Chile
2Facultad de Ingeniería y Arquitectura, Universidad Católica de El Salvador, By pass a Metapán y carretera Antigua a San Salvador, El Salvador
In Santa Ana, Department of El Salvador, there is an accession of coffee derived from Bourbon, which has been sheltered for several generations, enjoying resistance to this disease and maintaining cup quality. The objectives of the work were to establish a micropropagation protocol for this variety, as well as to perform the molecular characterization of the vitroplants obtained. Mature seeds were used, disinfected and introduced into a sterile solution of boric acid and cysteine. The culture medium was an MS (pH 5.7) with 0.5 mg L-1 of 6- BAP. For multiplication, MS was used with the macrosalts at half the initial concentration, supplemented with 1.5 mg L-1 of 6-BAP and 30 g L-1 of sucrose. The multiplication rates varied between 2.1 and 4.5 with statistical differences. For the phase of development and rooting the MS culture medium was used with two treatments: one, without hormone with the macrosalts at half the concentration, and another, of the same formulation plus 2.5 mg L-1 of ANA and 10 g L -1 of sucrose; both kept in darkness for 14 days. For the indicators of number and length of roots in the phase of development and rooting, the formulation medium MS without hormone (T1), presented statistical differences, so it is proposed for the propagation of this accession of coffee. The statistical analysis was Analysis of Variance, with comparison of means by Tukey. For the determination of the genetic fingerprint, microsatellite markers or repeated simple sequences (SSRs) were used; establishing that all the resulting plants have the same genetic profile.
Key words: climate change; genetic stability; microsatellites; resistance
INTRODUCTION
Coffee cultivation (Coffea arabica L.) has traditionally been one of the most important economic items in El Salvador for its contribution to the Gross Domestic Product (GDP), as well as for the generation of employment and its contribution to the conservation of biodiversity 1.
Currently, both in El Salvador and throughout the world, the change in climatic conditions is causing the deterioration of natural ecosystems due to the increase in temperature, the absence or excess of rainfall, etc. 2,3, directly or indirectly affecting the loss of wild flora and fauna, which may lead to the generation of genetic resistance to pests and diseases, especially in commercial crops. This is the specific case of rust (Hemileia vastatrix), a fungus that has coffee as an obligatory host and grows on the underside of the leaves, where it forms circles of orange or dusty yellow, causing chlorotic lesions affecting the photosynthetic function, breathing and perspiration and that in the last two years has devastatingly affected commercial coffee plantations in the Central American area 3-5. In these countries, accumulated losses of USD $ 243 million are reported due to rust in the years 2012 to 2014. In El Salvador the losses have been around USD $ 25.9 million 6. The most affected varieties are Arabic, which are those that provide coffee with the best cup quality and are the most cultivated in the country and Latin America; However, due to the genetic narrowness caused by originating from a few seeds brought from Ethiopia, problems such as loss of resistance against some diseases such as rust arise 6,7.
Other factors also affect coffee production are price fluctuations in the international market; National economic policies and reduction of crop areas. All this has caused that the productivity and volume of exports have experienced considerable decreases.
Due to the aforementioned, it is essential to look for strategies that allow reproducing materials that show resistance or tolerance to rust in the field, maintaining cup quality, which will improve crop yields. In this regard, there is an accession of coffee derived from the Bourbon variety, produced on a farm in the coffee zone of Santa Ana municipality from the Department of the Western Zone of El Salvador; which has been protected for several generations and can offer a solution to these eventualities. Despite lacking the genetic profile of this accession, rust resistance has remained in the field for a long time even during the height of the rust attack, which has been corroborated by agricultural technical personnel and coffee growers in the area.
In addition to this context, the technique of plant micropropagation is a viable alternative for the reproduction of elite plants on a large scale and in a short period of time, also guaranteeing the health of the reproduced material. On the other hand, molecular characterization by microsatellite markers is a useful tool that allows to verify the genetic stability of micropropagated plant materials.
Coffee is a very important crop for the national economy of El Salvador, and for the first time we have worked on the establishment of a micropropagation protocol, which contributes to the rapid multiplication of plants with genetic quality, so that the objectives of the work were to establish a micropropagation protocol for the accession of Bourbon coffee under study, as well as to perform the molecular characterization of the vitroplants with microsatellite markers or repeated simple sequences (SSRs), with a view to demonstrating the genetic affinity between them.
MATERIALS AND METHODS
In the UNICAES Plant Tissue Cultivation laboratory, coffee seeds (Coffea arabica L.) of a protected accession of the Bourbon variety were used, which were introduced in 70 % alcohol for 5 minutes. Subsequently, they were subjected to 50 % commercial chlorine disinfection (5.25 % NaClO) plus two drops of Tween 80 for 15 minutes. Then they were taken to laminar flow to perform three rinses with sterile water. After this process, the seeds were introduced into a sterile solution of boric acid (0.5 %) and cysteine (25 mg L-1) 8, and incubated for three days in dark conditions. After this time the solution was removed, rinsed three times with sterile water and the embryos were carefully removed using tweezers and scalpel.
The culture medium used for the embryo establishment phase was an MS 9 supplemented with 0.5 mg L-1 of 6-BAP (6-N-Benzylaminopurine), with a pH of 5.7. The containers used were glass bottles with a capacity of 190 mL, applying a volume of 28 mL of culture medium to each. Sterilization was performed in autoclave at 121 °C and 15 pounds of pressure/in2, for 15 minutes. When the embryos that were in the establishment phase germinated, they were transferred to light conditions in the growth room. The photoperiod of incubation was 16 hours of light and 8 hours of darkness, with 1350 lux intensity, with an average temperature of 26 °C, the duration of this stage was 23 days. For the multiplication stage, the MS medium was used with half macrosalt, supplemented with 1.5 mg L-1 of 6-BAP and 30 g L-1 of sucrose. For this stage, the germinated plants of approximately one month were cut into sections and placed vertically in the culture medium; this process was continued approximately once every 25-30 days for at least five cycles. For the development and rooting phase, the MS culture medium was used with two different treatments: one, which is the same formulation of the multiplication medium, but without the hormone 6-BAP (T1), and another, also with macrosalt at half plus 2.5 mg L-1 of auxin ANA (naphthalenacetic acid) and 10 g L-1 sucrose (T2); remaining in these conditions and in darkness for a period of 14 days. This was done to favor the hormonal stimulation of the plant and start root formation. After this time, these plants were placed in MS medium of previous formulation (MS culture medium with macrosalts in half and without 6-BAP hormone).
Each of the stages or phases of the micropropagation process, specifically the multiplication, lasted approximately 30 days, depending on how long the plants had two or three internodes ready for cutting. The experimental design used was Completely Random with five repetitions. The variables evaluated were the number of shoots per explant in the multiplication phase; while the height of the plants (cm), the number of leaf pairs, and the number of plants that survived and the length of the roots (cm), were evaluated in the development and rooting phase. The statistical analysis of the data was Analysis of Variance (ANOVA), with comparison of means by Tukey (p≤0.01). The statistical program Statgraphics Centurion XVI.I.
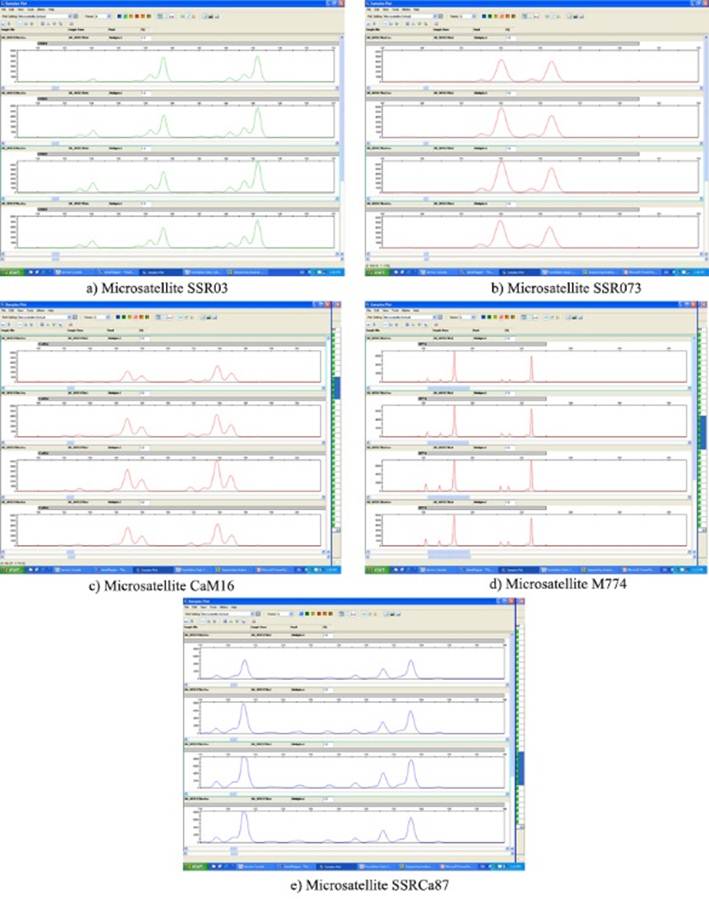
The stability of the micropropagated plants was assessed by means of the DNA fingerprint, which was obtained with microsatellite markers or repeated simple sequences (SSRs) 10. A subset of six vitroplants was selected, which were lyophilized and stored for the duration of the analysis. Of these plants, 40 mg of the leaves were used to extract the DNA using a CTAB-based method 11. The extracted DNA was visualized on a 1 % agarose gel and quantified by spectrophotometry (NanoDrop, ThermoScientific, USA); DNA was adjusted to 50 ng µL-1 for microsatellite PCR. The DNA of each of the six samples was amplified with 10 microsatellites (Table 1) that have shown polymorphism for the genus Coffea 11-16.
Each PCR reaction had the following components in final concentration: ~ 100 ng of DNA extracted, 1X of Master Mix Maxima Hot Start PCR (ThermoScientific, USA) which contains the DNA polymerase, the final concentration at 200 µM of each dNTP and 2 mM of magnesium chloride. Each primer had a final concentration of 0.2 µM, the fluorochromic overtaking primer being. The PCR reaction was carried out in a final volume of 25 µL. The thermocycling program was initially denatured at 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, with a final extension of 72 ° C for eight minutes. The PCR products were visualized by capillary electrophoresis in an ABI 3130xl device (Applied Biosystems, USA) in a 50 cm capillary and in the POP7 matrix. The fragment size was obtained with the GeneMapper 4.0 program, using the GeneScan LIZ600® internal molecular mass marker as a reference.
Tabla 1 Microsatellite markers and primers used in the analysis of the genetic stability of the micropropagated coffee material (Coffea arabica)
| Name of microsatellite | Sequence of the overtaking primer (5’ - 3’) | Reverse primer sequence (5’ - 3’) | Reference |
| SSR04 | GGTCGCTCACTCATATCTTCCAG | GCCTGGAAAGCAAACGTCTCA | 12 |
| SSRCa087 | TCACTCTCGCAGACACACTAC | GCAGAGATGATCACAAGTCC | 15 |
| M753 | GGAGACGCAGGTGGTAGAAG | TCGAGAAGTCTTGGGGTGTT | 14 |
| SSRCa088 | TACCTCTCCTCCTCCTTCCT | ATTTCTATGGACCGGCAAC | 16 |
| CaM16 | AAGGCAGCTGAAGCGGGACAAA | TGGGGAGAGCTGCAGTTGGAGG | 14 |
| M24 | GGCTCGAGATATCTGTTTAG | TTTAATGGGCATAGGGTCC | 17 |
| M764 | CTGGCATTAGAAAGCACCTTG | GCTTGGCTCACTGTAGGACTG | 18 |
| M32 | AACTCTCCATTCCCGCATTC | CTGGGTTTTCTGTGTTCTCG | 17 |
| SSR03 | GGACAAAACACCGCCCAAAATA | AGCGAGACAGAGGAAGGGAATATT | 16 |
| SSR073 | GAGGTCTTCCCACCACAACA | GGATACGAGAGTCCCTTCC | 15 |
RESULTS
The germination of the embryos began after 13 days of sowing in the establishment phase and was completed after one month, when the vitroplants already had two pairs of true leaves. Then, the multiplication phase began, studying five cycles. The multiplication rates fluctuated with a decrease in cycle 2, followed by an increase in cycle 3 (with statistical differences p≤0.01, according to Tukey), to then decrease again in number 4, and from there, it seems stabilize with a tendency to greater fluctuations (Figure 1).
The vitroplants were always vigorous and with intense green coloration; each plant had four to six pairs of leaves each month, allowing a new cut. In this phase there was also spontaneous appearance of roots in most plants. It was also observed that in all the multiplication cycles it was necessary to change the culture media every 25 or 30 days, at which point the plants apparently depleted the nutrients causing the foliage to begin to become necrotic.
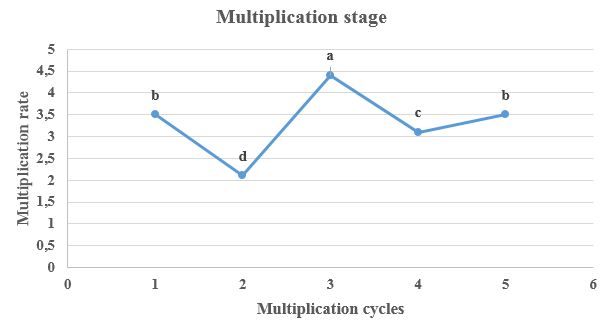
Figure 1 Multiplication rates (or number of shoots per explant) of coffee plants (Coffea arabica) corresponding to five cycles of multiplication under in vitro conditions (p≤0.01)
After six days of beginning the development and rooting phase, it was observed that the vitroplants of the ANA treatment and 10 g L-1 sucrose (T2) began to form more robust roots than the T1 treatment plants (Figure 2); however, they presented callus formation at the base of the plant. After a month of being in this development phase, the roots of these plants were more numerous and longer in the T1 treatment, in addition to having formed, in some cases, buds around the original explant.

A) MS medium without hormone and 30 g L-1 sucrose
B) MS medium plus 2.5 mg L-1 of ANA and 10 g L-1 of sucrose
Figure 2 Images of the final phase of in vitro rooting of a coffee accession (Coffea arabica) of the Bourbon variety, after 30 days, under two treatments
Table 2 shows the variables evaluated in this phase. In general, it is observed that the height of the plants showed a tendency to be greater in T1, without statistical differences. However, the number of roots and their length were greater in T2; with statistical differences.
Table 2 Result of the growth variables evaluated at the end of the development and rooting phase of coffee plants obtained in vitro, in two different culture media: T1- MS medium without hormone and 30 g L-1 sucrose, T2 - MS medium plus 2.5 mg L-1 of ANA and 10 g L-1 of sucrose
| Variables | Treatments | Mean | Signification(a) |
|---|---|---|---|
| Plant height (cm) | 1 | 2,52 | n.s. |
| 2 | 2,35 | ||
| Nu. of pairs of leaves | 1 | 3,92 | n.s. |
| 2 | 4,26 | ||
| No. Of roots | 1 | 1,17 | ** |
| 2 | 2,74 | ||
| Root length (cm) | 1 | 3,19 | ** |
| 2 | 5,97 |
a: n.s. = not significant; ** = significant differences according to the Tukey test (p≤0.01)
The results of the molecular study of 10 microsatellites in each of the coffee samples analyzed revealed the genetic stability of the micropropagated material (Figure 3). In this case, the results of the PCR products displayed as profiles by capillary electrophoresis are shown.
DISCUSSION
In the first place it can be mentioned that although vegetative propagation is complicated for Coffea arabica17, this method of obtaining the zygotic embryo adapted from the procedure performed at the INCA Biotechnology Laboratory (Cuba) 8 for robust varieties, facilitates the reproduction of some Arabic cultivars, including this accession derived from the Bourbon variety used for this trial. The use of this method of propagation might seem to lead to genetic variation; however, the study carried out with microsatellite molecular markers showed that the plants produced by this route are clones propagated from a single tissue donor plant (Figure 3) and therefore retain the same genetic characteristics between them.
According to Armendáriz 19, the main function of the multiplication stage is to increase the number of propagules in culture to increase the availability of plants. In this case, the multiplication was performed during five cycles and did not show a uniform behavior; since while one cycle increased the rate of sprout production, the next tended to decrease. However, it was observed that during the middle of the reproductive cycle there was an increase in production, reaching a rate of 4.5 (Figure 1). This situation could be due to the fact that the material has had greater vigor at that stage; since, according to Condori 20, with concentrations of 6- BAP between 1 or 2 mg L-1, the maximum number of outbreaks in juvenile materials is obtained. In this work it was obtained with a dose of 1.5 mg L-1. In spite of this, it is considered that the production of buds by explant in this way is lower than that produced by orthotropic or plagotropic meristems 21. In addition, some authors 22 recommends not carrying out beyond a fifth multiplication cycle, due to the considerable decrease in the number of outbreaks produced by explant.
In the development and rooting phase, the hormonal stroke method of treatment 2 for 14 days and then returning them to a means of hormonal deficiency, was used to produce stress in the plants, which in turn causes an acceleration in the metabolism and the development of new tissues to survive 23. However, in the case of T2, callus formation occurred at the base of the plants; not so in T1, which contained MS medium with half macrosalt, 30 g L-1 sucrose and no hormones. Precisely one of the effects of auxins, in addition to tissue elongation, is the formation of calluses at the base of the buds; this counteracts the result obtained by Dublin 24.
In T2, the appearance of the roots took more than seven days, but in the end they were more numerous and longer than those of T1, with significant differences (Table 2). On this aspect, it can also be noted that the dose of 6- BAP used in the medium did not inhibit the formation of roots in regenerated plants, since they spontaneously occurred from the multiplication phase; partly because the dose used was not so high, but 1.5 mg L-1.
In general, it can be said that the number and length of roots of the regenerated plants were favored by the presence of auxin ANA in the culture medium, since it is an excellent hormone in the induction of tissue elongation 8,25.
The microsatellite marker (SSRs) technique was used because of the advantage of being more reproducible than other molecular marker systems 26. Therefore, it is easy to establish assays to validate the genetic fingerprint data because the sequence of the primers is invariable and can be easily applied in different laboratories. It was observed that the six plant samples presented the same genotype for the set of 10 microsatellites used, which indicates that these samples have the same genetic profile between them and could show that they are clones propagated from a tissue donor plant. Although there is no genetic profile of the donor plant, the crop shows stability at the field level.
The analysis in GeneMapper showed that the analyzed samples have more than two different alleles for some microsatellites, with a maximum of four alleles and a minimum of two. The microsatellites are codominant markers, that is, for the same locus they can detect homozygous and heterozygous individuals. In the case of Coffea arabica, it means that in each copy of the genome individuals can have up to eight different alleles, in case each copy is heterozygous. Or if each copy is homozygous, four alleles will be observed.
In the case of microsatellites that amplified four alleles, it cannot be determined whether each copy of the genome is homozygous since the alleles have similar molecular weights. Or it could be that two copies are heterozygous for one set of alleles and another two copies are heterozygous for another set of alleles.
CONCLUSIONS
Finally, it can be said that based on the methodology used in this trial, the micropropagation of Arabic coffee is feasible, especially this accession of Bourbon coffee, which has been propagated by the sexual embryo from seed, and that its genetic stability it remains among the resulting plants, which was evidenced by the microsatellites used.
BIBLIOGRAFÍA
1. Bonilla T. Estadísticas | Consejo Salvadoreño Del Café [Internet]. 2019 [cited 09/05/2019]. Available from: http://www.csc.gob.sv/estadisticas/ [ Links ]
2. Virginio Filho E de M, Astorga Domian C. Prevención y control de la roya del café: manual de buenas prácticas para técnicos y facilitadores. Turrialba, Costa Rica: CATIE; 2015. [ Links ]
3. Flores M, Bratescu A, Martínez JO, Oviedo JA, Acosta A. Centroamérica: El impacto de la caída de los precios del café. CEPAL. Serie estudios y perspectivas. Sede Subregional México. 2002;(9):81. [ Links ]
4. Cano-Sánz CG, Vallejo-Mejía F-C, Caicedo-García E, Amador-Torres JS, Tique-Calderón EY. El mercado mundial del café y su impacto en Colombia. Borradores de Economía. 2012;(710):56. [ Links ]
5. SENASICA. Roya del cafeto (Hemileia vastatrix Berkeley & Broome) [Internet]. México, D.F.: Dirección General de Sanidad Vegetal. Programa de Vigilancia Epidemiológica Fitosanitaria; 2016 [cited 09/05/2019] p. 23. Report No.: 40. Available from: https://www.americaeconomia.com/negocios-industrias/perdidas-por-roya-del-cafe-ascienden-us243m-en-centroamerica [ Links ]
6. Xinhua. Pérdidas por roya del café ascienden a US$243M en Centroamérica [Internet]. Available from: https://www.americaeconomia.com/negocios-industrias/perdidas-por-roya-del-cafe-ascienden-us243m-en-centroamerica [ Links ]
7. Anzueto F. Variedades resistentes a roya [Internet]. Asociación Nacional del Café (Anacafé). 1985 Available from: https://www.anacafe.org/glifos/index.php/Variedades_resistentes_a_roya [ Links ]
8. Castilla Valdés Y. Conservación de recursos fitogenéticos de cafeto (Coffea spp.) por métodos biotecnológicos: una alternativa para su preservación. Cultivos Tropicales. 2012;33(4):29-39. [ Links ]
9. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum. 1962;15(3):473-97. [ Links ]
10. Azofeifa-Delgado Á. Uso de marcadores moleculares en plantas; aplicaciones en frutales del trópico. Agronomía mesoamericana. 2006:221-42. [ Links ]
11. Araya E, Murillo O, Aguilar G, Rocha O. A DNA extraction protocol and initial primers screenining in hieronima alchorneoides Fr. All. for aflp applications. Foresta Veracruzana. 2005;7(1):1-4. [ Links ]
12. Aggarwal RK, Hendre PS, Varshney RK, Bhat PR, Krishnakumar V, Singh L. Identification, characterization and utilization of EST-derived genic microsatellite markers for genome analyses of coffee and related species. Theoretical and Applied Genetics. 2007;114(2):359. [ Links ]
13. Poncet V, Hamon P, Minier J, Carasco C, Hamon S, Noirot M. SSR cross-amplification and variation within coffee trees (Coffea spp.). Genome. 2004;47(6):1071-81. [ Links ]
14. Hendre PS, Phanindranath R, Annapurna V, Lalremruata A, Aggarwal RK. Development of new genomic microsatellite markers from robusta coffee (Coffea canephora Pierre ex A. Froehner) showing broad cross-species transferability and utility in genetic studies. BMC plant biology. 2008;8(1):51. [ Links ]
15. Missio RF, Caixeta ET, Zambolim EM, Zambolim L, Sakiyama NS. Development and validation of SSR markers for Coffea arabica L. Embrapa Café-Artigo em periódico indexado (ALICE). 2009 [ Links ]
16. Missio RF, Caixeta ET, Zambolim EM, Pena GF, Ribeiro AP, Zambolim L, et al. Assessment of EST-SSR markers for genetic analisys on coffee. Bragantia. 2009;68(3):573-81. [ Links ]
17. Etienne H, Dechamp E, Barry-Etienne D, Bertrand B. Bioreactors in coffee micropropagation. Brazilian Journal of Plant Physiology. 2006;18(1):45-54. [ Links ]
18. Sefc KM, Lopes MS, Mendonça D, Santos MRD, Machado MLDC, Machado ADC. Identification of microsatellite loci in olive (Olea europaea) and their characterization in Italian and Iberian olive trees. Molecular Ecology. 2000;9(8):1171-3. [ Links ]
19. Armendáriz Hidalgo KC. Establecimiento de un protocolo de desinfección, introducción y multiplicación in vitro a partir de yemas apicales de plantas juveniles de magnolia (magnolia grandiflora) para la producción masiva, repoblación en el Distrito Metropolitano de Quito [Ingeniería en Biotecnología]. [Sangolquí. Ecuador]: Universidad de las Fuerzas Armadas ESPE.; 2014. 128 p. [ Links ]
20. Condori Laurente K. Efectos de dosis de bencill amino purina (BAP) y el ácido naftalem acético (ANA) en la producción in vitro de estevia (Stevia rebaudiana B.) en Acobamba [Ingeniero Agróngmo]. [Acobamba-Huancavelica]: Universidad Nacional de Huancavelica; 2013. 79 p. [ Links ]
21. TefyPaho Ayala. Yemas de café [Internet]. 2013 [cited 09/05/2019]. Available from: https://es.slideshare.net/pahola_estefy/yemas-de-caf [ Links ]
22. Lozano K, Guillermo A. Propagación in vitro de café (Coffea arabica)-variedad Lempira-a partir de meristemas [Internet] [Ingeniero Agrónomo]. [Zamorano, Honduras]: Escuela Agrícola Panamericana; 2014. 31 p. Available from: http://bdigital.zamorano.edu/bitstream/11036/3482/1/CPA-2014-050.pdf [ Links ]
23. Pincay Bajaña VJ. Multiplicación in vitro de café caturra rojo Coffea arábica L. con la interación de dos fitohormonas [Internet] [Ingeniera Agrónoma]. [Guayaquil-Ecuador]: Facultad de Ciencias Agrarias Universidad de Guayaquil; 2017. 69 p. Available from: http://repositorio.ug.edu.ec/bitstream/redug/18266/1/Picay%20Baja%c3%b1a%20Vanessa%20Juliana.pdf [ Links ]
24. Dublin P. Multiplicación vegetativa de café, hevea y cacao. In: Roca W, Mroginski L, editors. Cultivo de tejidos en la Agricultura: Fundamentos y aplicaciones. Cali, Colombia: Centro Internacional de Agricultura Tropical-CIAT; 1991. p. 577-96. [ Links ]
25. Alvarenga S. Manual de Laboratorio de Cultivo de Tejidos I. Cartago, Costa Rica: Escuela de Biología. Instituto Tecnológico de Costa Rica; 1999. [ Links ]
26. Roa Delgado S, Fernández González H, Angulo Graterol L, Useche Carrillo N, De Faría Muñoz Y. Caracterización molecular de genotipos de Rubís mediante marcadores microsatélites. Agronomía Tropical. 2014;64(1-2):61-72. [ Links ]
Received: September 22, 2017; Accepted: May 13, 2019











 text in
text in