Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cultivos Tropicales
versión On-line ISSN 1819-4087
cultrop vol.40 no.2 La Habana abr.-jun. 2019 Epub 01-Jun-2019
Short Communications
Effect of culture medium pH on the presymbiotic growth of Rhizoglomus irregulare
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
2Facultad de Ingeniería en Biociencias, Universidad de Gent, Bélgica
Arbuscular mycorrhizal fungi (AMF) are represented in almost all terrestrial ecosystems and colonize more than 90 % of plants. Its association depends on many edaphoclimatic factors including pH one of the most important chemical parameters of the soil which directly regulates the availability of nutrients and can affect germination, diversity, spore density and colonization of roots by AMF The objective of this study was to evaluate the effect of the pH of the Strullu and Romand Modified culture medium (SRM) on the presymbiotic growth of Rhizoglomus irregulare (INCAM 11) on in vitro conditions. For the development of the experiment, surface sterilized AMF spores were exposed to different pH conditions (4.5, 5.5, 6.5 and 7.5). The growth of the germinative tube was evaluated during 4 weeks, presenting the higher values on pH 7.5. This result is within the range of optimum pH recommended for this strain in field experiments, in which it shows its highest efficiency.
Key words: mycorrhizae; germination of the spores; in vitro culture
INTRODUCTION
Arbuscular mycorrhizal fungi (AMF, Phyllum Glomeromycota) are an integral part of numerous ecosystems and are considered particularly advantageous because they are associated with most vascular plants 1. This mutualistic symbiosis facilitates the uptake of mineral nutrients and water while protecting plants against different diseases 2,3. Most AMF contribute to the stability of soil aggregates 4 and adapt to a wide spectrum of edaphic conditions that are related to their development and effectiveness 5,6. The pH is considered one of the most important chemical parameters of the soil 7, due to the effect it exerts both on the physical, chemical and biological characteristics of the soil, as well as on the crop yield. Through ion exchange the pH directly regulates the availability of nutrients, which determines the richness of species and the composition of the microorganism community among which are the AMF 7.
The pH determines in many cases the efficiency of the endophyte, the germination percentage and the development of the AMF spores 6,7. Therefore, it is very important in studies of the selection of AMF species, with high symbiotic efficiency, to take into account the effect of pH, either on the productivity of the association or on the fungal reproduction mechanisms, in order to be able to select the most efficient species or ecotypes in a wide range of pH or in the ranges that are of interest 8,9. Despite the importance of AMF fungi in the physiology and nutrition of plants, as well as in the formation of plant communities, the factors that affect germination, diversity, spore density and root colonization by AMF are poorly understood 8.
Based on the aspects described above, the objective of the study was to evaluate the effect of pH of the Strullu and Romand Modified (SRM) culture medium on the presymbiotic growth of Rhizoglomus irregulare (INCAM 11) under “in vitro” conditions.
MATERIALS AND METHODS
For the present study, the growth of the germ tube of Rhizoglomus irregulare (INCAM 11) was evaluated, under different pH conditions (4.5; 5.5; 6.5 and 7.5) of the Strullu and Romand Modified culture medium (SRM).
Biological material
For the execution of the experiment, INCAM11 spores were extracted from the HMA collection of the National Institute of Agricultural Sciences (INCA) of Cuba using the technique of wet and decanted sieving and subsequent extraction by centrifugation in sucrose gradient + Tween 80 at 2000 rpm in a centrifuge table (KUBOTA) for five minutes 10,11. Once the water-sucrose + Tween 80 interface was obtained, the spores were extracted using a 30 mL syringe.
Disinfection of Rhizoglomus irregulare sports. (INCAM - 11)
From the extracted propagules, spore clusters were selected according to the disinfection technique previously proposed by Cranenbrouck et al. 12, modified by Perera 13, which were placed on a membrane (0.44 μm pore) and washed three times with sterile distilled water. Subsequently, they were contacted with a 2 % Chloramine T solution and two drops of Tween 20 for 10 minutes. They were then washed three times with sterile distilled water and treated with a 10-minute antibiotic solution containing streptomycin sulfate (0.02 %) and gentamicin sulfate (0.01 %), which was sterilized with filter aid millipore (type HA, 4.0 cm in diameter and 0.22 μm pore). After this time the membrane with the propagules was transferred to the antibiotic solution, previously filtered in sterile Petri dish (90 mm diameter) for 24 hours.
Subsequently, the propagules were inoculated in 90 mm diameter Petri dishes, divided into 4 compartments each containing SRM culture medium with the different pHs under study 4,5; 5.5; 6.5 and 7.5 adjusted with HCL or NaOH as appropriate.
Assessment of the growth of the germinative tube of prohagles of rhizoglomus irregulare (INCAM-11) in in vitro conditions
This experiment was designed with the purpose of performing a dynamics of propagation germ tube of propagules of INCAM-11. This variable was evaluated from the moment in which germination tube growth was observed from the sporophore or the support hyphae. The measurements were made once a week for a month, with a micrometer coupled to the dissecting microscope (Novel, 40X magnification), starting from the beginning of the new hypha formed and to the apex thereof.
Statistic analysis
Once normality was verified, the confidence interval of the means was calculated at 95 % probability, according to the number of repetitions (10 replicates per treatment) and the reproducibility of the data. The means comparison analyzes and the determination of the confidence intervals were performed by the STATISTIC version 6.1 program.
RESULTS AND DISCUSSION
The effect of the different pHs on the growth of the germ tube of the Rhizoglomus irregulare strain (INCAM 11) is shown in Figure 1. It was observed that the highest germ tube length values were shown by the strain at pH 7.5, presenting statistically significant differences from the beginning of the experiment with the rest of the treatments.
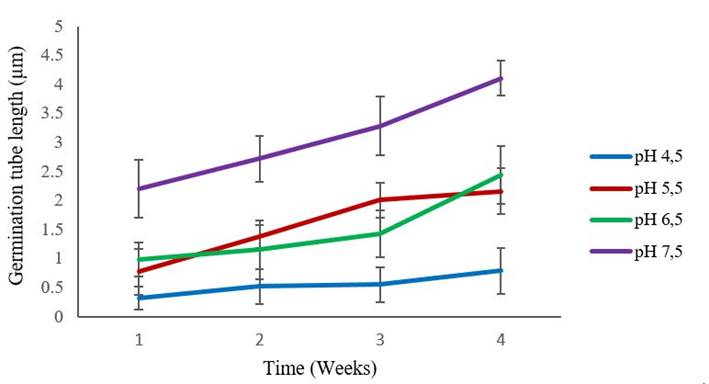 The bars represent the confidence intervals of the average of treatments for p≤ 0.05 (n = 4 treatments)
The bars represent the confidence intervals of the average of treatments for p≤ 0.05 (n = 4 treatments)Figure 1 Dynamics of the germ tube growth of INCAM 11 propagules inoculated in SRM medium for four weeks
Research aimed at the study of the effect of pH on mycorrhizal colonization, suggests that there are certain strains of AMF that are insensitive to the increase in pH (pH 7values), although these, in general, have better performance in alkaline soils, or in conditions pH neutral 7. Not obtaining the same results for acid soils where the number of hyphae and arbuscules present in the roots examined were scarce.
The National Institute of Agricultural Sciences (INCA) has been working since 1992 on the use and extension of these fungi in plant nutrition in general and in phosphorus in particular, with a system of recommendation of strains by type of soil, depending on of its pH, organic matter content and fertility level 14. In this way, studies carried out on Carbonated Fluffy Brown soils with a slightly alkaline pH-H2O 2,7 it was observed that the INCAM 11 strain always had a superior and significant effect (p≤0.05), differing from those obtained by the remaining strains Glomus cubense and Funneliformis mosseae15. In the present investigation, this result allowed us to confirm that the strain had the best germination values at a pH close to that used for the aforementioned extension study 15. This confirms that the effect of pH is decisive for the efficiency of the strain used by type of soil, which shows that its potentiality is related to the pH of the medium in which it develops. This allows you to access the infection site more easily which allows you to establish itself in these conditions and exert their effect.
Recently, some authors have stated that the relationship established between the pH ranges of the soil and the effect of mycorrhizal colonization is truly complex, depending not only on the fungal species, but also on the type of soil, the way in which find nutrients (mainly P and N and other elements such as Cu, Zn, Mo, B, etc.) and to a lesser extent the species of plant on which it develops 8, in this sense, it is noteworthy that these effects are important from the beginning of the symbiont life cycle, as observed in this experiment, which confirms the theory that optimal functioning is determined by the pH of the substrate on which the symbiont develops.
BIBLIOGRAFÍA
1. Ortiz N, Armada E, Duque E, Roldán A, Azcón R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. Journal of plant physiology. 2015;174:87-96. [ Links ]
2. Augé RM, Toler HD, Saxton AM. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza. 2015;25(1):13-24. [ Links ]
3. Sánchez-Romera B, Calvo-Polanco M, Ruiz-Lozano JM, Zamarreño ÁM, Arbona V, García-Mina JM, et al. Involvement of the def-1 mutation in the response of tomato plants to arbuscular mycorrhizal symbiosis under well-watered and drought conditions. Plant and Cell Physiology. 2017;59(2):248-261. [ Links ]
4. Wu Q-S, Cao M-Q, Zou Y-N, He X. Direct and indirect effects of glomalin, mycorrhizal hyphae, and roots on aggregate stability in rhizosphere of trifoliate orange. Scientific Reports. 2014;4:5823. doi:10.1038/srep05823 [ Links ]
5. Khan A, Sharif M, Ali A, Shah SNM, Mian IA, Wahid F, et al. Potential of AM fungi in phytoremediation of heavy metals and effect on yield of wheat crop. American Journal of Plant Sciences. 2014;5(11):1578-86. [ Links ]
6. Kanwal S, Bano A, Malik RN. Role of arbuscular mycorrhizal fungi in phytoremediation of heavy metals and effects on growth and biochemical activities of wheat (Triticum aestivum L.) plants in Zn contaminated soils. African Journal of Biotechnology. 2016;15(20):872-883. [ Links ]
7. Ouzounidou G, Skiada V, Papadopoulou KK, Stamatis N, Kavvadias V, Eleftheriadis E, et al. Effects of soil pH and arbuscular mycorrhiza (AM) inoculation on growth and chemical composition of chia (.Salvia hispanica L.) leaves. Brazilian Journal of Botany. 2015;38(3):487-95. doi:10.1007/s40415-015-0166-6 [ Links ]
8. Kawahara A, An G-H, Miyakawa S, Sonoda J, Ezawa T. Nestedness in Arbuscular Mycorrhizal Fungal Communities along Soil pH Gradients in Early Primary Succession: Acid-Tolerant Fungi Are pH Generalists. PLOS ONE. 2016;11(10):e0165035. doi:10.1371/journal.pone.0165035 [ Links ]
9. Vyas D, Gupta RK. Effect of edaphic factors on the diversity of VAM fungi. Trop Plant Res. 2014;1:14-25. [ Links ]
10. Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society. 1963;46(2):235-44. doi:10.1016/S0007-1536(63)80079-0 [ Links ]
11. Herrera RA, Ferrer RL, Furrazola E, Orozco MO. Estrategia de funcionamiento de las micorrizas VA en un bosque tropical. Biodiversidad en Iberoamérica. Ecosistemas, evolución y procesos sociales. Programa Iberoamericano deficiencia y tecnología para el desarrollo. Subprograma XII. Diversidad Biológica: Mérida. 1995. [ Links ]
12. Cranenbrouck S, Voets L, Bivort C, Renard L, Strullu D-G, Declerck S. Methodologies for in Vitro Cultivation of Arbuscular Mycorrhizal Fungi with Root Organs. In: Declerck S, Fortin JA, Strullu D-G, editors. In Vitro Culture of Mycorrhizas [Internet]. Berlin, Heidelberg: Springer; 2005 [cited 05/05/2019]. p. 341-75. doi:10.1007/3-540-27331-X_18 [ Links ]
13. Perera García SS. Cultivo in vitro de Glomussp13. . (INCAM 11) asociado a raíces transformadas de Cichoriumintybus.13. [Trabajo de Diploma]. Universidad de La Habana, Facultad de Biología. 2017. 32p. [ Links ]
14. Rivera R, Fernández F, Fernández K, Ruiz L, Sánchez C, Riera M, et al. Advances in the management of effective arbuscular mycorrhizal symbiosis in tropical ecosystesm. In: Ed. Hamel E, Plenchette C, editors. Mycorrizae in Croup Productions. 2007. p. 151-95. [ Links ]
15. João JP, Espinosa Cuellar A, Ruiz Martínez L, Simó González J, Rivera Espinosa R. Efectividad de cepas de HMA en el cultivo de la yuca (Manihot esculenta Crantz) en dos tipos de suelos. Cultivos Tropicales. 2016;37(1):48-56. [ Links ]
Received: December 26, 2018; Accepted: May 14, 2019











 texto en
texto en 


