Cultivos Tropicales
ISSN 1819-4087
01--2019
Short communication
Disinfection of pepper seeds (Capsicum annuum L.) cultivar ‘YAMIL’ for in vitro implantation
1Universidad Agraria de La Habana “Fructuoso Rodríguez Pérez”, carretera a Tapaste y Autopista Nacional, San José de las Lajas, Mayabeque, Cuba
2Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
3Centro de Investigaciones Científicas de Yucatán. Mérida. México
In the Department of Genetics and Plant Improvement of the National Institute of Agricultural Sciences, an experiment was carried out with pepper seeds (Capsicum annuum L.) cultivating ‘YAMIL’, in which different concentrations of sodium hypochlorite were compared ( NaOCl) [1.25; 2.5 and 5 %] and disinfection times (one, three and five minutes) of the pepper seeds cultivate ‘YAMIL’ for later implantation in vitro, in two culture media, which contained the salts of the basal culture medium of Murashige and Skoog (MS), supplemented with sucrose (15 and 30 g L-1), in order to determine the best treatment and disinfection time of the seeds of this pepper cultivar. The results obtained in this work indicated that 100% of the seeds were disinfected with 2.5 % NaOCl for three minutes or 5 % for three minutes. 100 % of the seeds germinated and the highest seedling height (5.84 cm), as well as the number of roots per seedling (5.15) and their vigor, showed the best results with the use of NaOCl (5 %) for one minute and the use of the culture medium MS supplemented with sucrose (30 g L-1) that were statistically superior to the control treatments. From the results obtained, a disinfection methodology is obtained for the in vitro implantation of the ‘YAMIL’ cultivar pepper.
Key words: contamination; germination; vegetable; sodium hypochlorite; sucrose
INTRODUCTION
The pepper is one of the most important horticultural species for Cuba and the world, belongs to the genus Capsicum, family Solanaceae. The genus Capsicum contains about 25 wild and five cultivated species, which are: Capsicum annuum L., Capsicum frutenscens L., Capsicum chínense Jacq, Capsicum bacatum L. and Capsicum pubescens R and P 1. It develops from near sea level to 2500 m a.s.l, covering different regions of Mexico and other parts of the world, which is why this crop could be found in the market all year round, so its consumption is very widespread in fresh and industrialized in various modalities 2,3.
From the cultivated species, Capsicum annuum L. is the one of greater economic importance, since it is widely consumed by the world population as a condiment. In addition, its fruits have medicinal properties, because they contain volatile oils, capsaisicinoids, carotenoids, vitamins, proteins, fibers, antioxidants and mineral elements 4-6. There are cultivars that differ in shape, size, color, flavor and culinary uses 7.
On the other hand, it has been seen that the use of explants for the regeneration of apical meristems 8, leaves, hypocotyls, cotyledons, roots and embryos induce somatic embryogenesis 9-11. However, not all cultivars respond equally to these techniques or to prior disinfection of explants, so adjustments and basic knowledge of the physiological mechanisms involved in the different stages of the micropropagation of a crop are needed 12.
‘YAMIL’, is a cultivar of open pollination, of a cycle of 130 days, presents good climatic adaptation, it is recommended for wintertime (September 15 to February 15). It has good foliage cover that allows the fruits to be protected from sunburn and predators. It begins to bloom at 29 days after transplantation (dat), its massive flowering occurs at 34 dat; the fruits are green that turn red at their physiological maturity, it is 9.94 cm long and 9.35 cm wide, as well as a thickness of 6.62 mm. The average mass of the fruit ranges between 200-230 g and has between six to seven fruits per plant. The yield is 30 t ha-1; acidity (0.16 %), °Brix (4.5), pH (5.5-5.6) and between 170-175 mg in 100 g of vitamin C content; it is resistant to Potyvirus. This genotype is registered in the official variety registry of the Ministry of Agriculture of Cuba 13.
Currently working on the genetic improvement of cultivating ‘YAMIL’ for high temperatures; on the one hand, since it is a genotype for open field and to improve the quality of the fruits; on the other hand, in terms of lycopene content. Therefore, obtaining seedlings of this crop in a shorter time is essential and biotechnological techniques must be used, so research should be initiated on the disinfection of seeds for in vitro implantation and decrease the improvement time with respect to traditional methods.
There is a wide range of chemical agents that are used in the disinfection of explants before in vitro inoculation, so it is essential to establish the type of disinfectant to be used, the time of treatments and concentrations, since the same way these act on the microorganisms, they do on the treated material and can cause irreparable damage 14).
The disinfection of the seeds of different genotypes of Capsicum spp. it was performed with 5 % sodium hypochlorite for 10 minutes 15. On the other hand, some authors, reported that adequate disinfection of the explants (embryo-endosperm structures) of moringa seeds (Moringa oleifera Lam.) was performed with 1 % sodium hypochlorite (v:v) under stirring for seven minutes (16. Calcium hypochlorite is also used at different concentrations and disinfection times, depending on the species and the cultivar. Likewise, mercury chloride II (HgCl2) is also used in the disinfection of explants to initiate in vitro culture in different plant species, but this disinfectant is very toxic, so it should be used with great caution.
Taking into account the above, this work was carried out with the objective of determining the best treatment of sodium hypochlorite and disinfection time of pepper seeds (Capsicum annuum L.) cultivar ‘YAMIL’ for in vitro implantation.
MATERIALS AND METHODS
The experiment was conducted in the Department of Genetics and Plant Improvement of the National Institute of Agricultural Sciences (INCA), Cuba.
Vegetal material
Certified pepper seeds (Capsicum annuum L.) from the ‘YAMIL’ cultivar of the 2016-2017 campaign were used.
Culture medium
The salts of Murashige and Skoog 17 [MS] were used as the basal culture medium. The combinations of culture media were the following:
The pH was adjusted to 5.8-0.2 before sterilizing it in an autoclave at 1.5 atmospheres of pressure and 121 °C for 20 minutes.
In all cases, 10 mL of culture medium per bottle will be used.
Growing conditions
All the flasks with the explants were placed in a growth chamber at a temperature of 24±2 ºC, at a flux density of photosynthetic photons between 220-250 µmol m-2 s-1, with a photoperiod of 16 light hours and eight of darkness and relative humidity between 70-75 %.
The treatments were as follows:
1. Running water + commercial detergent (0.5 g in 100 mL of solution) +inoculation in the basal culture medium MS + sucrose (15 g L-1)- Control.
2. Running water + commercial detergent (0.5 g in 100 mL of solution) +inoculation in the basal culture medium MS + sucrose (30 g L-1)- Control.
3. Running water +commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1).
4. Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1).
5. Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1).
6. Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1).
7. Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5 %) - 1 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1).
8. Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5 %) - 1 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1).
Evaluations
They were performed at 7, 14 and 21 days, but the results of the last evaluation will be presented at work. The variables evaluated were the following:
• Percentage of disinfection of seeds [explants]: the total number of seeds that were disinfected was determined, with respect to the total that were inoculated in the culture medium. Subsequently the percentage was determined.
• Seed germination percentage [explants]: the total number of seeds that germinated was determined with respect to the total that were inoculated in the culture medium. Subsequently the percentage was determined.
• Height of seedlings in vitro (cm): it was measured with a sterile millimeter paper that was in a Petri dish.
• Number of roots per seedling in vitro: the total roots of each seedling were counted per treatment and subsequently the average was obtained.
• Root length per seedling in vitro (cm): the same procedure was followed to measure seedling height.
• Vigor of seedlings in vitro: It was determined according to the scale proposed by Izquierdo et al. 18, where: 1- Not very vigorous; 2- Vigorous; 3- Very vigorous. This evaluation was performed by visual appreciation.
Experimental design and data analysis
A completely randomized design with 15 bottles was used per treatment with two seeds (explants) per bottle and it was repeated twice in time. The data were processed using Simple Classification Variance Analysis (ANOVA), with the SPSS 11.5 program for Windows (SPSS, Inc., Chicago, IL) and the comparison between the means was performed according to the Tukey test (p(0.05).
RESULTS AND DISCUSSION
The results related to the percentage of disinfection and germination of pepper seeds (explants) are reflected in Figure 1. As can be seen there were significant differences in the percentage of seed disinfection; 100 % of the seeds of treatments 6 and 8 were disinfected and there were no differences between the seeds of these treatments, but if they differed from those of treatments 1 and 2. It is important to clarify that the contamination of the seeds (explants) in the different treatments it was mainly with bacteria. Regarding the percentage of seed germination, only those of treatment 8 reached 100 % germination and were statistically differentiated from the rest of the treatments.
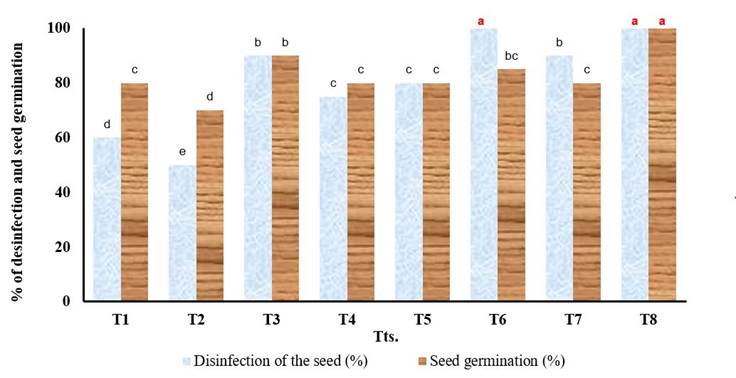 T1.- Running water + commercial detergent (0.5 g in 100 mL of solution) + inoculation in the basal culture medium MS + sucrose (15 g L-1) .- ControlT2.- Running water + commercial detergent (0.5 g in 100 mL of solution) + inoculation in the basal culture medium MS + sucrose (30 g L-1) .- ControlT3.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1)T4.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1)T5.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1)T6.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1)T7.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5 %) - 1 minute + inoculation in the basal culture medium MS + sucrose (15 g L-1)T8.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5%) - 1 minute + inoculation in the basal medium MS + sucrose (30 g L-1)n.- total seeds (explants) of the experiment in the two repetitions Tts.- treatmentsEEx.- standard error of the mean D.E.- standard deviationDifferent letters in the columns indicate significant differences between treatments for the Tukey test (p≤0.05)Means with different letters differ statistically according to the Tukey test (p≤0.05) (*** significant for p <0.001)
T1.- Running water + commercial detergent (0.5 g in 100 mL of solution) + inoculation in the basal culture medium MS + sucrose (15 g L-1) .- ControlT2.- Running water + commercial detergent (0.5 g in 100 mL of solution) + inoculation in the basal culture medium MS + sucrose (30 g L-1) .- ControlT3.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1)T4.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1)T5.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1)T6.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1)T7.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5 %) - 1 minute + inoculation in the basal culture medium MS + sucrose (15 g L-1)T8.- Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5%) - 1 minute + inoculation in the basal medium MS + sucrose (30 g L-1)n.- total seeds (explants) of the experiment in the two repetitions Tts.- treatmentsEEx.- standard error of the mean D.E.- standard deviationDifferent letters in the columns indicate significant differences between treatments for the Tukey test (p≤0.05)Means with different letters differ statistically according to the Tukey test (p≤0.05) (*** significant for p <0.001)Figure 1 Percentage of disinfection (EEx=3.25***; SD=4.10) and germination (EEx=2.90 ***; SD=3.00) of pepper seeds (explants) (Capsicum annuum L.) cultivar ‘YAMIL’ in culture media with different concentrations of sucrose, 21 days after inoculation in vitro. n=60
100 % of the pepper seeds of the cultivar ‘Jalapeño M’, were disinfected with 100 and 50 % of Clorox; however, the germination percentage was higher with the highest concentration (92.6 %) and the lowest affected it (64.2 %) 19.
Explants obtained from seeds of different genotypes of Capsicum spp., were disinfected with water containing Tween 20, 70 % ethanol and 5 % NaOCl for 10 minutes and obtained a high percentage of seed germination 15. On the other hand, Stanislava and Velichka 20 also obtained good results regarding the germination of the seeds of the pepper cultivars 'Yasen F1' and 'Kurtovskakapia 1619', when they were disinfected with a solution of calcium hypochlorite at 5 % for one hour.
Although calcium hypochlorite is a good disinfectant agent, it is possible that in the previous results, the percentage of germination of the seeds in both pepper cultivars has been slightly affected, since these seeds were exposed for a long time to the chemical agent.
In other crops such as Aloe vera (L.) Burm. f., Lavanya and Thayamini21, it was reported that the sprouts extracted from the mother plant were washed with tap water for approximately 40 minutes and then disinfected with 70 % ethanol for 30 seconds and then these explants were placed in 20 % Clorox (5.25 % NaOCl) with two drops of Tween 20 for 30 minutes. According to other authors 22, when disinfection was carried out for one minute in 96° alcohol and 20 minutes in 2 % NaOCl (5.6 g L-1 of active chlorine), with two drops of Tween 20, the percentage of in vitro contamination of Schinus fasciculata (Griseb) seeds JM Johnstvar. fasciculata (molle), implanted in agar, -water was 16 %. These authors also reported that the percentage of physiological germination three days after inoculation in the culture medium was 40 % and after seven days they reached 84 %.
Other authors disinfected basil seeds (Ocimum basilicum L.) with 10 % NaOCl for five minutes and 70 % ethanol for 20 seconds 23. In other investigations three methods were used for the disinfection of the seeds of Aristotelia chilensis (Molina) Stuntz 24, the first disinfection was carried out in a solution containing Mancozeb and Benomilo (2 g L-1 of Mancozeb; 0.6 g Benomil L-1, plus a few drops of Tween 20) for 20 minutes and placed on a magnetic stirrer; the second disinfection was with a 75 ° alcohol solution for five seconds and, finally, the seeds were immersed in 1 % NaOCl for 10 minutes.
Chemical fertilizers, such as Benomilo and Mancozeb, are very aggressive for use in the disinfection of explants in vitro, regardless of the results obtained, since they can be toxic and difficult to remove the explant in vitro, which is subsequently it will become a plant, consumed by people and animals and affect the environment.
The disinfection of the eggplant cultivars (Solanum melongena L.) 'Mattu Gulla' and 'Perampalli Gulla', were carried out by introducing their seeds in a soapy solution (two or three drops of Tween 20) for five minutes, followed by ethanol treatment 70 % for one minute and subsequently placed the seeds in mercury (II) chloride (HgCl2) 0.1 % (w/v) for five minutes 25. The germination of the seeds of Stenocereus queretaroensis (F. A. C. Weber) F. Buxb. (An arborescent cactus, endemic to Mexico), treated with 10 % sodium hypochlorite for five minutes, was superior to those that were not treated 26).
Sodium and calcium hypochlorite, as well as mercury (II) chloride cause the death of infectious microorganisms such as bacteria and exogenous fungi, which allow a higher rate of establishment in the plant material. However, the first two should be used at certain concentrations and disinfection times, since they are very potent and in the latter case, they should not be used in the disinfection of explants, since it is very toxic to them when grown in vitro and very difficult to remove from them.
Table 1 shows the height of the seedlings, the number and length of the roots, as well as the vigor of the seedlings and there were significant differences between the treatments in all the variables that were evaluated. With respect to the height of the seedlings, the best treatment was in which the seeds were placed in 5 % NaOCl for one minute and they were implanted in the MS culture medium supplemented with 30 g L-1 sucrose, with 5, 84 cm high of the seedling, which was statistically differentiated from the control treatments 1 (MS culture medium supplemented with 15 g L-1 sucrose) and 2 (MS culture medium supplemented with 30 g L-1 sucrose), which The seedlings reached 4.61 and 4.94 cm, respectively. The seedlings of treatment 8 also differed statistically from those of the rest of the treatments.
Table 1 Height (cm), number and length (cm) of the roots, as well as the vigor of pepper seedlings (Capsicum annuum L.) cultivar ‘YAMIL’ in culture media with different concentrations of sucrose, at 21 inoculated days in vitro
| No. | Treatments | Seedling height (cm)) | Number of roots per seedling (cm) | Root length (cm) | Seedling Vigor |
|---|---|---|---|---|---|
| 1 | Running water + commercial detergent (0.5 g in 100 mL of solution) + inoculation in the basal culture medium MS + sucrose (15 g L-1).- Control | 4,61 f | 3,75 c | 3,04 d | 2 b |
| 2 | Running water + commercial detergent (0.5 g in 100 mL of solution) + inoculation in the basal culture medium MS + sucrose (30 g L-1) .- Control | 4,94 de | 4,20 b | 3,48 c | 2 b |
| 3 | Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1) | 4,89 e | 4,35 b | 4,08 a | 2 b |
| 4 | Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (1.25 %) - 5 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1) | 5,00 d | 4,25 b | 3,09 d | 2 b |
| 5 | Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (15 g L-1) | 5,23 c | 4,35 b | 3,16 d | 2 b |
| 6 | Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (2.5 %) - 3 minutes + inoculation in the basal culture medium MS + sucrose (30 g L-1) | 5,38 b | 4,30 b | 3,94 a | 2 b |
| 7 | Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5 %) - 1 minute + inoculation in the basal culture medium MS + sucrose (15 g L-1) | 5,25 c | 4,60 b | 3,45 c | 2 b |
| 8 | Running water + commercial detergent (0.5 g in 100 mL of solution) + sodium hypochlorite (5 %) - 1 minute + inoculation in the basal culture medium MS + sucrose (30 g L-1) | 5,84 a | 5,15 a | 3,76 b | 3 a |
| E.Ex(() | 0,02*** | 0,10*** | 0,04*** | 0,00*** | |
| D.E | 0,36 | 0,57 | 0,41 | 0,33 | |
Different letters in the same column indicate significant differences between treatments for the Tukey test (p ≤ 0.05). n = 60
n.- total seedlings (explants) of the experiment in the two repetitions EEx.- standard error of the mean
D.E.- standard deviation
In relation to the number of roots, the treatment seedlings 8 were statistically superior to those of the rest of the treatments, including those of the control treatments (1 and 2). However, with respect to the length of the roots, the seedlings of treatments 3 and 6, with 4.08 and 3.94 cm, respectively achieved the best results (Table 1).
Finally, the most vigorous seedlings were those of treatment 8, which reached a vigor of 3 and exceeded those of control treatments 1 and 2, with a vigor of 2 in both cases (Table 1).
The seedlings of the 'Jalapeño M' cultivar, with 50 % Clorox applications, reached greater height and length of the cotyledon leaves unlike the seedlings in which 100 % Clorox was used. However, they did not report significant statistical differences with respect to the width of the cotyledon leaves or the length of the roots 19).
Other authors 27 in studies conducted with Escobaria cubensis (Britton and Rose) Hunts, commonly called “Holguin dwarf cactus”, reported that the treatment of the seeds with 2 % NaOCl and double disinfection, were those that had higher levels of seedling survival; the mammals responded better in terms of survival to the greater combinations of NaOCl concentration and the type of disinfection.
Obtaining different types of primary explants from three pineapple cultivars (Ananas comosus (L.) Merr.:crown yolks and the meristem of the grandfathers, from stems, which were previously washed with detergent and subsequently transferred to a diluted solution of commercial chlorine at 20 % (v/v) for 10 minutes, they observed that at four weeks of cultivation, there was an apical bud in all cultivars, approximately 3 to 4 mm long. After an additional four weeks in the same culture medium, there was elongation of the leaves and the stem (1 to 3 cm) and the elongation increased with time, without lateral sprouting being observed 28. In this work, the seedlings of treatment 8 (5 % NaOCl for one minute and inoculation of the seeds in the MS basal culture medium supplemented with sucrose (30 g L-1), showed a homogeneous development, given the high synchronization that it was observed in the g eradication of them. In addition, this material constituted an adequate source of quality explants for subsequent experiments.
From these results, a methodology of disinfection of pepper seeds is cultivar ‘YAMIL’ proposed for in vitro implantation.
Steps of the methodology:
1. Select pepper seeds (Capsicum annuum L.) cultivar ‘YAMIL’ that are certified and apparent symptoms of fungal or bacterial diseases.
2. Place the seeds in a 250 mL capacity beaker and rinse them with plenty of tap water and commercial detergent (0.5 g in 100 mL of solution).
3. Rinse the seeds with tap water until all foam from the detergent is removed.
4. In the laminar flow cabinet, the seeds are placed in sodium hypochlorite (5 %) for one minute and two drops of Tween 80 per 100 mL of solution are added and stirred.
5. Decant the solution and rinse the seeds four times with sterile distilled water.
6. Place the seeds in a Petri dish containing sterile filter paper to remove moisture in them.
7. Inoculate two seeds per bottle, containing 10 mL of MS culture medium supplemented with 30 g L-1 sucrose.
CONCLUSIONS
• An adequate disinfection methodology was for the in vitro implantation of pepper seeds (Capsicum annuum L.) established to cultivar ‘YAMIL’, which guarantees the germination of the seeds (explants), as well as the adequate survival and growth of the seeds seedlings.
• Seed inoculation should be carried out in a glass jar, containing 10 mL of the MS basal culture medium supplemented with sucrose (30 g L-1).
BIBLIOGRAFÍA
1. Resources IB for PG. Genetic resources of capsicum: a global plan of action. IBPGR Secretariat; 1983. [ Links ]
2. Latournerie ML, Aguilar RVH, López LP, Ramírez MS, Corona TT, López ST, et al. Los recursos genéticos del chile Capsicum spp. México: estudio, conservación y utilización. Resumenes Ejecutivos Ejercicio. 2010;157-9. [ Links ]
3. Palma J, Corpas FJ, Ruíz C, Molina T, Campos-Ramos MJ, Juanena A, et al. Los pimientos de las variedades Padrón, Piquillo, y Alegría riojana son una buena fuente de macro y microelementos para nuestra dieta [Internet]. Interempresas. 2016 [cited 21/06/2019]. Available from: https://www.interempresas.net/Horticola/Articulos/154064-pimientos-variedades-Padron-Piquillo-Alegria-riojana-son-buena-fuente-macro.html3. [ Links ]
4. Bosland PW, Votava EJ. International CAB. Peppers?: vegetable and spice capsicums [Internet]. Wallingford, Oxon, UK?; New York?: Cabi; 2000 [cited 21/06/2019]. Available from: https://trove.nla.gov.au/version/462430144. [ Links ]
5. Srinivasan K. Antioxidant potential of spices and their active constituents. Critical reviews in food science and nutrition. 2014;54(3):352-72. [ Links ]
6. Palma JM, Sevilla F, Jiménez A, del Río LA, Corpas FJ, Álvarez de Morales P, et al. Physiology of pepper fruit and the metabolism of antioxidants: chloroplasts, mitochondria and peroxisomes. Annals of botany. 2015;116(4):627-36. [ Links ]
7. Rodríguez Ruiz M. Dinámica de los antioxidantes en la maduración y post-cosecha de pimiento capsicum annuum l.) [Internet] [http://purl.org/dc/dcmitype/Text7. ]. Universidad de Granada; 2017 [cited 21/06/2019]. Available from: https://dialnet.unirioja.es/servlet/tesis?codigo=1101147. [ Links ]
8. Christopher T, Rajam MV. Effect of genotype, explant and medium onin vitro regeneration of red pepper. Plant Cell, Tissue and Organ Culture. 1996;46(3):245-50. [ Links ]
9. Agrawal S, Chandra N, Kothari SL. Plant regeneration in tissue cultures of pepper Capsicum annuum L. cv. Mathania). Plant cell, tissue and organ culture. 1989;16(1):47-55. [ Links ]
10. Kintzios S, Drossopoulos JB, Lymperopoulos C. Effect of vitamins and inorganic micronutrients on callus growth and somatic embryogenesis from young mature leaves of rose. Journal of plant nutrition. 2000;23(10):1407-20. [ Links ]
11. Savita A, Pati PK, Virk GS, Nagpal AK. An Efficient Somatic Embryogenesis Protocol for Citrus jambhiri and Assessment of Clonal Fidelity of Plantlets Using RAPD Markers. Journal of Plant Growth Regulation. 2014;34:309-19. doi:10.1007/s00344-014-9465-6 [ Links ]
12. Pishbin N, Mousavi A, Kalatejari S, Shariatpanahi M, Jahromi BB. The effect of plant growth regulators and different types of explants on in vitro regeneration of sweet pepper Capsicum annuum L.). International Journal of Biosciences (IJB). 2014;5(5):139-46. [ Links ]
13. Rodríguez Y, Depestre T, Rodríguez SR, Camejo SM, Salgado JM, Hernández A, et al. Nuevas variedades de pimiento para campo abierto con buena calidad del fruto, resistentes a Potyvirus. Revista Agrotecnia, Cuba. 2015;39(2). [ Links ]
14. Aniel Kumar O, Rupavathi T, Subba Tata S. Adventitious shoot bud induction in chili pepper Capsicum annuum L. cv. X-235). International Journal of Science and Nature. 2012;3(1):192-6. [ Links ]
15. Orlinska M, Nowaczyk P. In vitro plant regeneration of 4 Capsicum spp. genotypes using different explant types. Turkish Journal of Biology. 2015;39(1):60-8. [ Links ]
16. Matos Ruiz A, Capote Betancourt I, Pérez Martínez A, Lezcano Más Y, Aragón Abreu CE, Pina Morgado D, et al. Propagación in vitro de cultivares de Moringa oleifera Lam. Cultivos Tropicales. 2016;37:49-56. [ Links ]
17. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum. 1962;15(3):473-97. [ Links ]
18. Izquierdo Oviedo H, Alcaraz Meléndez L, Rodríguez-Álvarez M. Micropropagación de chiltepín Capsicum annuum L. cv.'glabriusculum') mediante el empleo de una oligosacarina de origen péctico. Acta universitaria. 2017;27(5):34-43. [ Links ]
19. Calderón Aguirre C. Evaluación in vitro del brasinoesteroide BB-6 y del oligosacárido Pectimorf en Capsicum annuum L. variedad jalapeño M. [Internet] [Maestría]. [Facultad de Ciencias Biológicas y Agropecuarias]; 2013 [cited 21/06/2019]. 99 p. Available from: https://cdigital.uv.mx/19. [ Links ]
20. Grozeva S, Todorova V. In vitro regeneration in pepper Capsicum annuum L.) and characterization of plant-regenerants. Electronic Journal of Biology. 2015;11(1):17-22. [ Links ]
21. Lavakumaran L, Seran TH. Effect of 6-benzyl-aminopurine and thidiazuron on in vitro shoot organogenesis of Aloe vera (L.) Burm. f. Chilean journal of agricultural research. 2014;74(4):497-501. [ Links ]
22. Freire R, Carnevale NJ, Alzugaray C, Bueno MS. Cultivo in vitro de Schinus fasciculata (Griseb) JM Johnst var. fasciculata (molle). Revista Colombiana de Biotecnología. 2014;16(2):169-73. [ Links ]
23. Guerra-Cantú JA, Moreno-Limón S, Cárdenas-Ávila ML, González-Luna AR, Salcedo-Martínez SM, Sánchez-Sánchez AA. Efecto del estrés osmótico sobre el desarrollo in vitro de plántulas de albahaca OcimumbasilicumL. Universidad Autónoma de Nuevo León. 2016;1(1):207-13. [ Links ]
24. Rodríguez Beraud M, Tampe Pérez J, Hormazábal Vásquez N, Araneda Durán X, Tighe Neira R, Cárcamo-Fincheira P. Efecto de la escarificación y estratificación sobre la germinación in vitro de Aristotelia chilensis (Molina) Stuntz. Gayana. Botánica. 2017;74(2):282-7. [ Links ]
25. Muthusamy A, Vidya KS, Pratibha PK, Rao MR, Vidhu SB, Guruprasad KP, et al. Establishment of an in vitro plantlet regeneration protocol for unique varieties of brinjal Solanum melongena L.) var. Mattu Gulla and Perampalli Gulla. 2014; [ Links ]
26. Solís-Márquez O, Plascencia-Escalante FO, Romero-Manzanares A, Cruz-Rodríguez JA, Ángeles-Pérez G, López-Acosta JC, et al. Potencial reproductivo de Stenocerus queretaroensis Cactaceae de San José de Cosalima, Zacatecas. Revista mexicana de biodiversidad. 2018;89(2):553-62. [ Links ]
27. Rodríguez de Francisco LE, Daquinta MA, Fornet Hernández E, Cantillo Ardeból R, Vásquez J. Propagación in vitro de escobaria cubensis (Britton Rose) Hunts. Ciencia y sociedad. 2013;38(2):345-75. [ Links ]
28. Rivas MAM, Mosquera HRM, Medina CLA. Micropropagación clonal y enraizamiento ex vitro de tres cultivares de piña Ananas comosus (L. Merr.) del Chocó, Colombia. Revista Biodiversidad Neotropical. 2014;4(2):133-40. [ Links ]
Received: December 06, 2018; Accepted: July 05, 2019















