My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Cultivos Tropicales
Print version ISSN 0258-5936On-line version ISSN 1819-4087
cultrop vol.40 no.4 La Habana Oct.-Dec. 2019 Epub Dec 01, 2019
Original article
Pectimorf® and Azofert-F® in the growth of bean plants (Phaseolus vulgaris L.)
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
In recent years in agriculture, the use of biostimulants has been enhanced to improve productivity and crop quality. The objective of this work was to determine the most effective concentration of a mixture of oligogalacturonides (Pectimorf®) in combination with the inoculant Azofert® (based on rhizobia), in the vegetative development of bean plants (Phaseolus vulgaris L.) Cuba Cueto variety -25-9-N. Two experiments were conducted under controlled conditions, in which two forms of application were evaluated and the concentrations 1, 5, 10, 20, 40 and 100 mg L-1 of Pectimorf® in seeds inoculated with Azofert® at the concentration of 1x109 CFU mL-1, and a dose of 200 mL of Azofert® per 46.04 kg of seed. In the first experiment the seeds were treated with the mixture of Azofert® and Pectimorf® prior to planting and in the second experiment, the foliar spray of Pectimorf® in the vegetative stage V3 was evaluated. Stimulation of nodulation and growth with the joint application of biostimulants depended on the form of application and the concentration of Pectimorf®. Most of the morphoagronomic indicators evaluated were stimulated with foliar spray of Pectimorf® at concentrations of 1, 5 and 100 mg L-1. However, only the radical dry mass was stimulated with 10 mg L-1 Pectimorf® when applied to the inoculated seeds.
Key words: biostimulants; legumes; development; nodulation
INTRODUCTION
Biostimulants are microorganisms or substances that favor plant nutrition, confer tolerance to abiotic stress and increase crop yield and quality 1,2. Microbial biostimulants, such as rhizobia-based inoculants are frequently used to decrease the use of inorganic fertilizers. These microorganisms have the ability to carry out biological nitrogen fixation (FBN) in symbiosis with leguminous plants and through this process; they supply the plant with atmospheric nitrogen in an assimilable form, thus favoring its growth and development 3.
Legumes are very variable in terms of their efficiency to fix atmospheric nitrogen in symbiosis, especially common beans (Phaseolus vulgaris L.), which is considered among the least studied in this process 4. For this reason, we work in the search for non-microbial biostimulants that, together with these bacteria, allow activating the physiology of these plants and increasing the yields and quality of the crops 5.
Among the non-microbial biostimulants most used in agriculture are the Oligosaccharines. Within some molecules the most studied for their biological applications are oligogalacturonids, chitins and chitosans and nodulation factors of the Rhizobiaceae family 6. Oligogalacturonides (OGAs) can regulate the synthesis and action of hormones and different organogenesis and growth processes in plants 7.
The mixture of OGAs, commercially called Pectimorf®, stimulates rooting, growth and cell differentiation of different plant species 6,8. In addition, it can activate defense mechanisms and reduce or mitigate environmental stress in plants 6,9,10. However, despite the use of Pectimorf® in several species in different concentrations, forms of application and growing conditions, a study has not yet been carried out to determine which the concentrations that favor the growth of beans are. For this reason the objective this work was to determine the concentration of Pectimorf® in combination with Rhizobium more effective in stimulating the nodulation and the growth of bean plants (Phaseolus vulgaris L.), by means of seed treatment and foliar spray.
MATERIALS AND METHODS
The study was at the National Institute of Agricultural Sciences conducted in Mayabeque Province, Cuba. As biostimulants, the commercial inoculant Azofert-F® of Rhizobium leguminosarum (CF1, 1x109 CFU mL-1) was used at a dose of 200 mL per 46.04 kg of seed and a mixture of peptide oligosaccharides (oligogalacturonides) known commercially as Pectimorf®
Seed application
Bean seeds of the Cuba C-25-9-N variety (40 seeds per treatment) were treated with 170 µL of a mixture containing Pectimorf® in different concentrations (1, 5, 10, 20, 40 and 100 mg L-1) and the Azofert-F® inoculant. The seeds were in containers of 0.55 kg capacity sown, with typical Leachate Red Ferralitic soil, eutric 11. Eight treatments were established: two controls (one inoculated with Azofert-F® (inoculated control (IC)) and the other not inoculated or treated with Pectimorf® (absolute control (CA)) and the rest corresponded to each of the concentrations of Pectimorf® used. The plants grew in a light room with 16 light hours, at a temperature of 25-27 ºC, with 60-70 % relative humidity and were every day with running water irrigated. In the growth, stage R5 when 50 % of the plants had the first buttons were evaluated the number and dry mass of the total nodules (g), the number of trifoliate leaves, the diameter and length of the stem (cm). Besides, the radical length (cm), the aerial and root biomass (g) and the relative content of total chlorophylls in the third trifoliate sheet (SPAD units) were evaluated using the MINOLTA SPAD 502 Plus portable meter.
Foliar spray application
Eight treatments were established two controls (one inoculated with Azofert-F® (CI) and the other not inoculated or treated with Pectimorf® (CA)) and the rest corresponded to each of the Pectimorf® concentrations used. The seeds were inoculated with Azofert-F® and when 50 % of the plants had the second pair of trifoliate leaves deployed (growth stage V3), they were sprinkled with 1.5 mL of Pectimorf® per plant at different concentrations. The experiment was performed under the same conditions as the previous one; the same variables were evaluated in the growth stage R5.
Experimental design and statistical analysis
The experiments were performed under a Fully Randomized Design with two repetitions and 20 plants were evaluated per treatment. All results were processed from a simple classification variance analysis and comparison of means by the Tukey test p <0.05 in the SPSS statistical program, Statistics v22. To verify the normality of the data, the Kolmogorov-Smirnov test was used and for the homogeneity of variances, the Levene statistic was used.
RESULTS AND DISCUSSION
Seed Application
The effect of the joint application of Pectimorf® and Azofert-F® to bean seeds at different concentrations is observed in Tables 1 and 2.
Table 1 Effect of application to seeds of Pectimorf® (Pm) and Azofert-F®
| Treatments | N.T | MSNT (g) |
|---|---|---|
| CA | 49.0 ab | 0.056 a |
| CI | 51.4 ab | 0.056 a |
| 1 mg L-1 Pm + A | 46.5 ab | 0.046 ab |
| 5 mg L-1 Pm + A | 39.1 b | 0.040 b |
| 10 mg L-1 Pm + A | 59.7 a | 0.055 a |
| 20 mg L-1 Pm + A | 43.9 b | 0.057 a |
| 40 mg L-1 Pm + A | 53.4 ab | 0.054 a |
| 100 mg L-1 Pm + A | 60.3 a | 0.061 a |
| ESx | 3.55 | 0.004 |
(A) in the nodulation of bean plants (Cuba Cueto-25-9-N) in stage R5. N.T: total nodules, MSNT: dry mass of total nodules. CA: absolute control, CI: inoculated control
Equal letters do not differ significantly for p <0.05. ESx, standard error of the mean
Table 2 Effect of application to seeds of Pectimorf® (Pm) and Azofert-F®
| Treatments | N. Leaves | L. Stem (cm) | L. Root (cm) | D. Stem (mm) | MSPA (g) | Chlorophylls (SPAD units) |
|---|---|---|---|---|---|---|
| CA | 4.93 | 57.82 | 27.32 ab | 2.82 | 0.86 | 33.57 b |
| CI | 4.73 | 68.00 | 26.33 ab | 2.74 | 0.96 | 36.64 ab |
| 1 mg L-1 Pm + A | 5.00 | 62.64 | 25.59 ab | 2.81 | 0.94 | 34.23 b |
| 5 mg L-1 Pm + A | 4.47 | 57.58 | 26.87 ab | 2.71 | 1.00 | 36.64 ab |
| 10 mg L-1 Pm + A | 4.60 | 58.46 | 24.55 b | 2.68 | 0.93 | 39.28 a |
| 20 mg L-1 Pm + A | 4.93 | 66.03 | 27.67 a | 2.74 | 0.97 | 33.51 b |
| 40 mg L-1 Pm + A | 5.10 | 61.99 | 25.08 ab | 2.67 | 0.85 | 33.67 b |
| 100 mg L-1 Pm + A | 4.73 | 63.03 | 24.54 b | 2.74 | 0.94 | 33.01 b |
| ESx | 0.22 (NS) | 2.83 (NS) | 0.7 | 0.05 (NS) | 0.05 (NS) | 0.99 |
(A) in the growth of bean plants (Cuba Cueto-25-9-N) in stage R5. N. Leaves: number of leaves, L. Size: stem length, L. Root: root length, D. Size: stem diameter, MSPA: dry mass of the aerial part, Chlorophylls: relative content of chlorophylls in the third leaf trifoliate. CA: absolute control, CI: inoculated control.
Equal letters do not differ significantly for p <0.05. ESx, standard error of the mean
In the nodulation variables evaluated, the effectiveness of the Azofert-F® inoculant was not evidenced with respect to the native population of the Rhizobium bacteria present in the soil used. Although the plants treated with Pectimorf® showed no significant differences in relation to the controls, there were differences between treatments (Table 1). Plants treated with concentrations of 10 and 100 mg L-1 had a greater number of nodules than plants treated with concentrations of 5 and 20 mg L-1. In the case of the dry mass of the nodules, it was lower for the treatment of 5 mg L-1 than for the rest of the treatments, this result is not sufficient to state that the application of the product at this concentration affects the biological fixation of nitrogen.
As for the growth variables, the highest value in the relative content of total chlorophylls in the third leaflet trifoliate corresponded to the plants treated with the concentration of 10 mg L-1, although this effect was not significant, compared to the control inoculated and with the treated plants with the concentration of 5 mg L-1.
The combination of both products increased the dry mass of the root in the treated plants with the concentration of 10 mg L-1, observing significant differences with the rest of the treatments, except with 5 mg L-1 (Figure 1). Although there were no significant differences in root length, the lowest value corresponded to plants treated with 10 mg L-1 Pectimorf®. These results indicate that this increase in dry root mass is due to an increase in the number of secondary and adventitious roots and not to the length of the root.
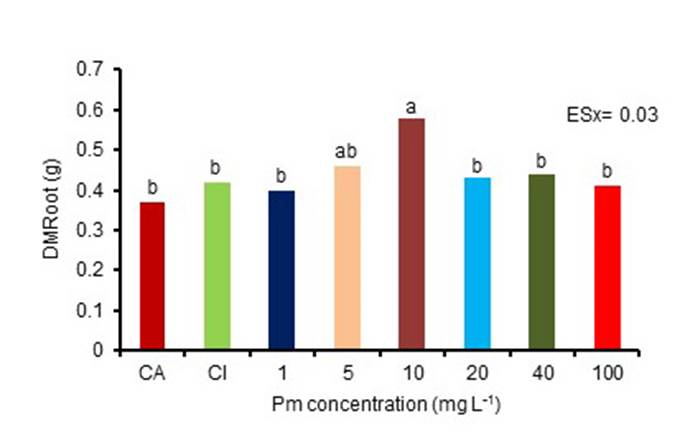
CA: absolute control, CI: inoculated control
Equal letters do not differ significantly for p <0.05. ESx, standard error of the mean
Figure 1 Effect of the application to seeds of Pectimorf® (Pm) and Azofert-F® in the dry root mass (DMRoot) of bean plants (Cuba Cueto-25-9-N), in stage R5
Similar results were obtained in experiments with soy plants (Glycine max) var. INCAsoy-24 inoculated with Bradyrhizobium and treated with 10 mg L-1 of Pectimorf® before sowing. The development of the radical system in the flowering stage, 60 days after sowing, was favored with the application of Pectimorf®, both in inoculated and non-inoculated seeds 12. Both results show the rooting power that some authors attribute to this mixture of OGAs 13-15.
In investigations conducted in petioles of African violet (Saintpaulia ionantha) with Pectimorf® at the concentration of 10 mg L-1, an increase in root length was evident, but not in the formation of secondary roots, an effect that was more significant than with the application of indole acetic acid (AIA) 13. This effect of Pectimorf® has also been obtained at concentrations below 10 mg L-1 (2 mg L-1) in banana plants cultivating ‘FHIA-18’ under in vitro conditions 16. In addition, at higher concentrations (20 mg L-1) in guava cuttings (Psidium guajava) var. Enana Roja Cubana, in which the rooting power of this product was manifested in the adventitious root formation 17.
These results corroborate what has been reported by other authors that OGAs can act as a substitute for traditional hormones, favoring cell differentiation and the formation of organs in plants 13.
Foliar spray application
Pectimorf® applied by foliar spray at the concentration of 100 mg L-1 stimulated the number of leaves produced, the dry mass of the aerial part, and the dry mass of the root and decreased the radical length. The rooting effect of the product was also observed at concentrations of 1 and 5 mg L-1. No effect of the products on the stem length was observed; however, the concentration of 1 mg L-1 increased its diameter (Table 3).
Table 3 Effect of foliar application of Pectimorf® (Pm) on the growth of bean plants (Cuba Cueto-25-9-N) inoculated with Azofert-F®
| Treatments | N. Leaves | L. Stem (cm) | L. Root (cm) | D. Stem (mm) | MSPA (g) | DMRoot (g) | Chlorophylls (SPAD units) |
|---|---|---|---|---|---|---|---|
| CA | 5.40 bc | 69.61 | 31.18 ab | 2.29 bc | 0.8704 b | 0.2553 c | 31.19 |
| CI | 5.47 bc | 75.50 | 31.62 ab | 2.21 cd | 0.8179 b | 0.2823 c | 33.94 |
| A+1 mg L-1 Pm | 5.20 c | 67.38 | 34.79 a | 2.53 a | 0.9998 ab | 0.3889 ab | 34.65 |
| A+5 mg L-1 Pm | 5.73 abc | 74.16 | 29.49 bc | 2.34 bc | 0.9704 ab | 0.4311 a | 32.36 |
| A+10 mg L-1 Pm | 5.53 bc | 74.68 | 26.52 cd | 2.32 bc | 0.9022 b | 0.3068 bc | 33.44 |
| A +20 mg L-1 Pm | 5.60 bc | 77.78 | 31.17 ab | 2.16 c | 0.9059 b | 0.2674 c | 32.61 |
| A +40 mg L-1 Pm | 6.07 ab | 80.13 | 25.08 d | 2.11 c | 0.9349 b | 0.3286 bc | 33.36 |
| A +100 mg L-1 Pm | 6.47 a | 81.11 | 24.06 d | 2.31 bc | 1.1442 a | 0.4229 a | 31.16 |
| ESx | 0.24 | 4.84 (NS) | 1.29 | 0.03 | 0.06 | 0.02 | 1.19 (NS) |
(A), in stage R5. N. Leaves: number of leaves, L. Root: root length, D. Size: stem diameter, MSPA: dry mass of the aerial part, MSRoot: dry mass of the root, Chlorophylls: relative content of chlorophylls in the third leaf trifoliate. CA: absolute control, CI: inoculated control.
Equal letters do not differ significantly for p <0.05. ESx, standard error of the mean
The relative content of total chlorophylls in the third trifoliate leaf did not show significant differences between treatments. There are authors who suggest that a possible way by which OGAs increase plant growth is because these molecules stimulate photosynthetic activity; which causes a greater gain of carbon skeletons that can be used for protein synthesis 18,19. However, it must be taken into account that photosynthetic activity is not determined solely by the chlorophyll content, but there are also other elements such as stomatal opening and closing and the activity of the rest of the components of the photosystems that influence in the process of photosynthesis.
In a study in bean plants sprinkled with 10 mg L-1 of Pectimorf®, in the second stage of the vegetative phase of cultivation, the product caused changes in distribution patterns and stomatal morphogenesis with an increase in the stomatal index. These modifications were fundamentally in the abaxial surface evidenced, where the occlusive cells were narrower and shorter. Likewise, it was observed that the leaves with the highest stomatal index had smaller stomata. The lower location of the stomata on the adaxial surface causes an increase in the stomatal resistance of this surface, when it is directly to solar radiation exposed and thus prevents water loss, which favors the availability of this substance for the photolysis to occur, a phenomenon that together with light is necessary for plants to carry out photosynthesis 20.
As for the nodulation variables, the number of total nodules was higher in the plants sprinkled with 100 mg L-1 and the dry mass of the nodules increased with the concentrations of 5 and 100 mg L-1 (Figure 2).
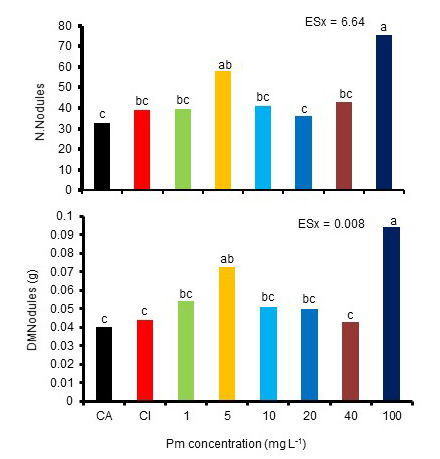
(A), in stage R5. CA: absolute control, CI: inoculated control
Equal letters do not differ significantly for p <0.05. ESx, standard error of the mean
Figure 2 Effect of the foliar application of Pectimorf® (Pm) on the number of total nodules (N. Nodules) and on the dry mass of the nodules (DMNodules) of bean plants (Cuba Cueto-25-9-N) inoculated with Azofert-F®
Background of the combination of both biostimulants is only in field conditions taken, in the cultivation of soybean var. INCAsoy-24 and INCAsoy-27. In both studies the plants inoculated with Bradyrihzobium were sprinkled at the beginning of flowering with Pectimorf® at the concentration of 10 mg L-1. The evaluations were performed 60 days after planting for INCAsoy-24 and 45 days for INCAsoy-27. In the case of INCAsoy-24, the application of both products increased the aerial and root dry mass, while in the INCAsoy-27 variety, only the aerial dry mass. In these experiments, the application of both products did not favor nodulation 12,21. However, in our case the evaluations were made 35 days after sowing, being found that at lower concentrations (1 and 5 mg L-1) and higher (100 mg L-1) than those applied in soy, Pectimorf® favored both the formation of secondary roots and the development of the nodules.
The increase in the dry mass of the root caused by Pectimorf® at 5 and 100 mg L-1 confirms what was stated by other authors, that this product, based on OGAs has auxinic activity 22-24, although it is also possible The function you perform is to activate signals that stimulate the synthesis of this hormone. To affirm this, biochemical and molecular studies are required.
It is proposed that the development of the radical system allows the plant, both under normal conditions of growth and drought stress, a greater absorption of water and minerals that allows an increase in foliar development 25, as shown in the results with the concentration of 100 mg L-1. The results let the development of the root allowed the plant an increase in the number of leaves and the dry mass of the aerial part.
The best results in the aerial part were with the application of 100 mg L-1 of Pectimorf®, obtained, a concentration that also increased the number of nodules. This result could be a consequence of the increase in the biological fixation of nitrogen that favors a greater synthesis of ureides, which allows the plant to have nitrogen for the synthesis of proteins and other molecules necessary for the formation and growth of the stem.
These increases in morphoagronomic variables and nodulation at concentrations of 1, 5 and 100 mg L-1 of Pectimorf® may be the result of a group of biological signals triggered in plants by the combination of these products. For this, it is recommended to carry out biochemical and molecular studies through the analysis of metabolites and enzymes involved in the metabolism of nitrogen and carbon that explain this effect. It is also proposed to evaluate these bioproducts in yield and crop quality.
CONCLUSIONS
The positive effects that Pectimorf® exerts, in combination with Azofert-F®, on the nodulation and growth of bean plants, depends on the form of application and the concentration of this oligosaccharin.
These macromolecules have a significant effect on root formation at concentrations of 10 mg L-1, when applied to seeds prior to planting and 1, 5 and 100 mg L-1 when applied by foliar spray.
The application of this mixture of OGAs to the seeds does not affect the nodulation. However, by foliar application at concentrations of 5 and 100 mg L-1 this process benefits.
Despite knowing that OGAs can act as substitutes for traditional hormones and stimulate plant growth and development, it is necessary to conduct studies that identify which processes related to vegetative growth are stimulated and how they benefit the biological fixation of nitrogen.
BIBLIOGRAFÍA
1. du Jardin P. Plant biostimulants: Definition, concept, main categories and regulation. Scientia Horticulturae. 2015;196:3-14. doi:10.1016/j.scienta.2015.09.021 [ Links ]
2. Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. Biostimulants in Plant Science: A Global Perspective. Frontiers in Plant Science. 2017;7:2049. doi:10.3389/fpls.2016.02049 [ Links ]
3. Zaccardelli M, Pentangelo A, Tripodi P. Characterization of Bean Phaseolus vulgaris L.) Ecotype "Fagiolo Occhio Nero Di Oliveto Citra" Using Agronomic, Biochemical and Molecular Approaches. Pakistan Journal of Biological Sciences. 2013;16(18):901-10. [ Links ]
4. Torres Gutiérrez R. Phytostimulatory effect of Rhizobium and Plant Growth Promoting Rhizobacteria in common bean Phaseolus vulgaris L.) interaction. 2008;155. [ Links ]
5. Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant and soil. 2014;383(1-2)3-41. [ Links ]
6. Falcón Rodríguez AB, Costales Mené D, González-Peña Fundora D, Nápoles García MC. Nuevos productos naturales para la agricultura: las oligosacarinas. Cultivos Tropicales. 2015;36:111-29. [ Links ]
7. Fundora LB, Ortiz RMH, Salcés ED, Gutiérrez MIR, Arencibia CG, Álvarez AR, et al. Embriogénesis somática de Citrus macrophylla Wester con el empleo del Pectimorf(r) y análogos de brasinoesteroides. Revista Colombiana de Biotecnología. 2013;15(1):189-94. [ Links ]
8. Falcón-Rodríguez AB, Costales D, Rogers HJ, Diosdado E, González S, Cabrera G, et al. Practical use of oligosaccharins in agriculture. Acta Horticulturae 2013;1009:195-212. [ Links ]
9. Terry Alfonso E, Ruiz Padrón J, Tejeda Peraza T, Reynaldo Escobar I. Efectividad agrobiológica del producto bioactivo Pectimorf(r) en el cultivo del Rábano Raphanus sativus L.). Cultivos Tropicales. 2014;35(2):105-11. [ Links ]
10. Terry-Alfonso E, Ruiz-Padrón J, Tejeda-Peraza T, Reynaldo-Escobar I, Carrillo-Sosa Y, Morales-Morales HA. Interacción de bioproductos como alternativas para la producción horticultura cubana. Tecnociencia Chihuahua. 2014;8(3):163-74. [ Links ]
11. Hernández JA, Pérez JJM, Bosch ID, Castro SN. Clasificación de los suelos de Cuba. Mayabeque, Cuba: Ediciones INCA. 2015;93. [ Links ]
12. Corbera J, Nápoles MC. Evaluación de la inoculación conjunta Bradyrhizobium japonicum-hongos MA y la aplicación de un bioestimulador del crecimiento vegetal en soya, cultivada en época de primavera. Cultivos Tropicales. 2011;32(4):13-9. [ Links ]
13. Falcón AB, Cabrera JC. Actividad enraizadora de una mezcla de oligogalacturónidos en pecíolos de violeta africana Saintpaulia ionantha. Cultivos Tropicales. 2007;28(2):87-90. [ Links ]
14. Alvarez Bello I, Reynaldo Escobar I, Cartaya Rubio O, Teheran Z. Efectos de una mezcla de oligogalacturónidos en la morfología de hortalizas de importancia económica. Cultivos Tropicales. 2011;32(3):69-74. [ Links ]
15. Pérez J, Aranguren M, Luzbet R, Reynaldo I, Rodríguez J. Aportes a la producción intensiva de plantas de guayabo Psidium guajava L.) a partir de esquejes en los viveros comerciales. CitriFrut. 2013;30(2):11-6. [ Links ]
16. García MB, Avalos DMR, Acosta JMZ, Batista RD. Efecto de Pectimorf(r) en el enraizamiento in vitro de plantas de 'FHIA-18'(Musa AAAB). Biotecnología Vegetal. 2015;15(4):227-32. [ Links ]
17. Ramos Hernández L, Arozarena Daza NJ, Lescaille Acosta J, García Cisneros F, Tamayo Aguilar Y, Castañeda Hidalgo E, et al. Dosis de pectimorf(r) para enraizamiento de esquejes de guayaba var. Enana Roja Cubana. Revista mexicana de ciencias agrícolas. 2013;4:1093-105. [ Links ]
18. El-Sharkawy MA. Utility of basic research in plant/crop physiology in relation to crop improvement: a review and a personal account. Brazilian Journal of Plant Physiology. 2006;18(4):419-46. [ Links ]
19. Ojeda CM. Efecto de un producto bioactivo compuesto por oligogalacturónidos como mitigador del estrés hídrico en variedades de albahaca (Ocimum basilicum L.) [Tesis de Doctorado]. [La Paz, Baja California Sur, México]: Centro de Investigaciones Biológicas del Noroeste, S.C.; 2015. 47-123. [ Links ]
20. Álvarez Bello I, Reynaldo Escobar IM. Efecto del Pectimorf(r) en el índice estomático de plantas de frijol Phaseolus vulgaris L.). Cultivos Tropicales. 2015;36(3):82-7. [ Links ]
21. Corbera Gorotiza J, Nápoles García MC. Efecto de la inoculación conjunta Bradyrhizobium elkanii-hongos MA y la aplicación de un bioestimulador del crecimiento vegetal en soya Glycine max (L.) Merrill), cultivar INCAsoy-27. Cultivos Tropicales. 2013;34(2):05-11. [ Links ]
22. Borges-García M, González-Paneque O, Reyes-Avalos DM, Rodríguez-González M, Villavicencio-Ramírez A, Abeal EE. Respuesta de plantas in vitro de ñame clon Blanco de guinea al uso del Pectimorf(r). Cultivos Tropicales. 2017;38(2):129-36. [ Links ]
23. Posada-Pérez L, Padrón-Montesinos Y, González-Olmedo J, Rodríguez-Sánchez R, Barbón-Rodriguez R, Norman-Montenegro O, et al. Efecto del Pectimorf(r) en el enraizamiento y la aclimatización in vitro de brotes de papaya Carica papaya L.) cultivar Maradol Roja. Cultivos Tropicales. 2016;37(3):50-9. [ Links ]
24. Suárez L. Efectos del Pectimorf(r) en la propagación in vitro de la yuca (Manihot esculenta C.), clones CMC-40 y Señorita [Tesis de Doctorado]. [Mayabeque]. Instituto Nacional de Ciencias Agrícolas; 2016. 50-54 p [ Links ]
25. Dell Amico J, Morales D, Jerez E, Rodríguez P, Álvarez I, Martín R, et al. Efecto de dos variantes de riego y aplicaciones foliares de Pectimorf(r) en el desarrollo del frijol Phaseolus vulgaris L.). Cultivos Tropicales. 2017;38(3):129-34. [ Links ]
Received: November 12, 2018; Accepted: July 02, 2019











 text in
text in 


