My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Cultivos Tropicales
Print version ISSN 0258-5936On-line version ISSN 1819-4087
cultrop vol.41 no.4 La Habana Oct.-Dec. 2020 Epub Dec 01, 2020
Original article
Effect of bioactive products on the germination of cryopreserved teak pollen grains
1Doctorado en Ciencias Naturales para el Desarrollo (DOCINADE), Instituto Tecnológico de Costa Rica, Universidad Nacional, Universidad Estatal a Distancia, Costa Rica
2Instituto de Investigación y Servicios Forestales (INISEFOR), Universidad Nacional, Apartado postal 86-3000, Heredia, Costa Rica
3Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
4Tecnológico de Costa Rica, Escuela de Ingeniería Forestal (ITCR), Cartago, Costa Rica
Teak (Tectona grandis L. f.), is a native tree from Southeast Asia considered of great economic value, mainly for its wood. As part of the efforts for the genetic improvement of this specie, protocols for pollen cryopreservation have been developed; however, it is essential to ensure its viability and efficient germination for future crosses of selected material. The objective of the present work was to evaluate the effect of spirostanic analogue of brassinosteroids (Biobras-16®) and a mixture of oligogalacturonides (Pectimorf®) on the in vitro germination, size of pollen and pollen tube length of cryopreserved teak pollen. The experiments consisted of evaluating the germination of pollen with 0, 4 and 8 months of cryopreservation in culture medium without supplements, supplemented with 0.001 and 0.050 Biobras-16® mg L-1 and, Pectimorf® 5 and 10 mg L-1. Germination data were analyzed using a General Linear Model, while pollen tube length and pollen area using analysis of variance (ANOVA) and correlation analysis. It was demonstrated that both bioactive products promote in vitro germination of cryopreserved pollen grains for 4 and 8 months, where Biobras-16® at 0.001 mg L-1 showed results that are more consistent. Pectimorf® stimulated the elongation of the pollen tube, which could favor the fecundation processes.
Key words: brassinosteroids; forestry; oligogalacturonides; Tectona grandis
INTRODUCTION
Teak (Tectona grandis) is a forest species native to Southeast Asia, whose wood is one of the best valued and best known in the world for its intrinsic qualities, which has prompted the development of genetic improvement programs to enhance its attributes 1,2. In the first stage of these programs, elite material has been selected, with high value commercial characteristics; however, it is necessary to advance towards the development of controlled crosses between genotypes to achieve greater progress in the quality and performance of this species. Both for the reproductive process and for genetic improvement from controlled crosses, it is necessary to guarantee the availability of viable pollen, to ensure the success of pollinations and the efficiency of improvement. Therefore, it is essential to know the germination capacity of the pollen grain and in this way, provide an estimate of possible female and male parents 3,4.
In vitro germination tests allow determining the amount of viable pollen and the growth of the pollen tube under controlled conditions, since the culture medium resembles the composition of the mucilage of the stigma 4. As part of this technique, in recent years the applications of bioactive substances that influence the growth and cell differentiation of various plant species have been studied, including brassinosteriodes (Br) and oligosaccharins (OG) 5.
Brassinosteroids are plant steroidal hormones, which have been found mainly in pollen, leaves, buds, flowers and seeds, in different proportions and forms 6. These compounds have been shown to have a positive effect on morphogenesis both in vitro and ex vitro, their response depends on the type and concentration used, as well as its interaction with plant hormones. Among these substances, Biobras-16® has been widely used, and has proven to be active at extremely low concentrations, generally solutions of 10-2 and 10-4 mg L-1 (7,8.
Oligogalacturonides are by enzymatic hydrolysis generated of the plant cell wall and at low concentrations, they show biological activity. These compounds are part of the most studied oligosaccharins; they are biostimulants considered because they are bioactive molecules whose function is to improve the physicochemical properties of plants, the yields and the quality of crops 9. The active ingredient in Pectimorf® is a mixture of pectic oligosaccharides, obtained from residues of the citrus industry; it includes important signaling molecules in the physiological processes of plants, related to growth and the stimulation of defense mechanisms. The optimal concentrations of the product to obtain a satisfactory biological response range between 10 and 20 mg L-1 (10,11.
The use of bioactive substances such as those mentioned can increase the germination efficiency of pollen grains and thus their fertility. The present study was with the objective of evaluating the effect of two Cuban products carried out, Biobras-16® and Pectimorf® on the in vitro germination of cryopreserved teak pollen.
MATERIALS AND METHODS
Trees established in clonal trials of the Novelteak Company, Guanacaste, Costa Rica were selected. Unopened flowers were collected from the inflorescences and they were placed in hermetically sealed plastic bags for one hour, to induce their opening. Once the flowers had been opened and with the help of dissection forceps, the anthers were mechanically extracted, which were placed in cryotubes.
For cryopreservation, the anthers with the pollen were frozen by direct immersion of the cryotubes in liquid nitrogen at -196 ºC for four and eight months. Once the respective storage time had elapsed, the samples were thawed at room temperature for five minutes and rehydrated in a humidity chamber for one hour 12.
The pollen grains contained in 0.1 g of anthers were detached from them by vibration, using a vortex, and placed in 2.5 mL cryovials for germination. 250 μL of liquid culture medium composed of Brewbaker and Kwack salts (BK) diluted to 10 % were added to each vial: Ca (NO3)2 300 mg L-1, MgSO4 200 mg L-1, H3BO3 100 mg L-1, KNO3 100 mg L-1 (13, as well as 10 g L-1 of sucrose and a final pH of 6.5. Each treatment was supplemented with the concentrations of Biobras-16® and Pectimorf®, as indicated in Table 1 and the non-supplemented medium was used as control.
Table 1 Treatments used for germination of teak pollen grains at 0, 4 and 8 months of cryopreservation
| Germination media | Supplements |
|---|---|
| M1 | None |
| M2 | 0.001 mg L-1 Biobras-16® |
| M3 | 0.050 mg L-1 Biobras-16® |
| M4 | 5 mg L-1 Pectimorf® |
| M5 | 10 mg L-1 Pectimorf® |
All treatments were for two hours at room temperature incubated and then 250 µL of acetocarmine (1 %) was as a dye added. The germination percentage was determined by the quotient of the number of germinated grains among the totals found in each microscopic field, considering germinated only grains with a pollen tube of length greater than or equal to the diameter of the pollen grain. Said counts were made using samples of 100 pollen grains per optical field, in a Nikon ALPHAPHOT-2 YS2 microscope (10x).
The measurements corresponding to the area of the pollen grain (mm2) and length of the pollen tube (µm) in fresh pollen samples were carried out using a Nikon eclipse 80i microscope with 20x magnification, coupled to a photographic camera with computer visualization through of the Nikon Ds-Fi-L2 program.
Regarding the experimental design for the pollen germination variable, a completely randomized design was carried out, with a factorial arrangement: culture medium (with five levels) and cryopreservation time (with three levels). On the other hand, for the variables area of the pollen grain and length of the pollen tube, a complete random design was carried out. Three repetitions and five readings per repetition were carried out in all treatments.
For statistical analysis, compliance with the statistical assumptions for parametric tests was verified and germination was evaluated using a General Linear Model, in order to calculate the effect of each factor and the interaction of both. The data corresponding to the area of the pollen grain and the length of the pollen tube were subjected to an analysis of variance (ANOVA) with multiple Tukey comparisons and to a linear correlation analysis by calculating the Pearson coefficient. All statistical tests were performed with 95 % confidence and using the Minab 19® statistical program.
RESULTADOS Y DISCUSIÓN
With the analysis of the data using the General Linear Model, it was shown with 95 % confidence that both the culture medium and the cryopreservation time and the interaction between both factors exerted a significant effect on the germination of teak pollen, all with a value of p = 0.000.
The control treatment (M1) showed a decrease in pollen germination at four months of cryopreservation, which was maintained at eight months. The treatment with Pectimorf® did not show a favorable effect for the germination of cryopreserved pollen, according to the behavior of treatments M4 and M5 (Figure 1).
The decrease in germination in cryopreserved pollen could be due to the genotype, the quality of the pollen and the amount of its nutrient reserves, caused by the environmental conditions where the genetic material used in the research was produced 14. In addition, it is known that cryopreservation can cause accumulation of reactive oxygen species and low temperature stress, the latter creating a disorganization of the plant's metabolism, causing alterations in the stability of proteins, enzymatic reactions and respiratory activity of cells 15,16.
On the contrary, the medium with brassinosteroid at 0.001 mg L-1 (M2) favored the germination of cryopreserved pollen for four and eight months, while the concentration of 0.05 mg L-1 of this compound showed a positive effect on the germination at four months. These results coincide with recent publications that report the use of brassinosteroids as growth promoters, reducers of oxidative damage caused by abiotic factors, and promoters of pollen germination of various species 7,10,15.

Different letters indicate statistically significant differences (Tukey, P≤0.05). M1: Without supplements, M2: 0.001 mg L-1 Biobras-16®, M3: 0.050 mg L-1 Biobras-16®, M4: 5 mg L-1 Pectimorf®, M5: 10 mg L-1 Pectimorf®
Figure 1 Effect of Biobras-16® and Pectimorf® on the germination of teak pollen cryopreserved for 0, 4, 8 months
When evaluating the germination of pollen without cryopreservation (0 months), there were significant differences between the treatments; those supplemented with Pectimorf® (M4 and M5) did not differ from the control (M1), while the treatments with Biobras-16® (M2 and M3) decreased germination at this time with respect to the control, the latter showing a germination percentage higher (33.5 %). This indicates that, in fresh pollen, the evaluated products did not achieve a positive effect on germination.
In the case of pollen conserved in liquid nitrogen for four months, a stimulatory effect of the brassinosteroid was observed in both concentrations evaluated, which presented significant differences with the rest of the treatments. The samples treated with Pectimorf® did not differ from the control.
After eight months of conservation, the lower concentration (0.001 mg L-1) of brassinosteroid showed a greater stimulus in the germination of teak pollen grains, with results superior to the rest of the treatments, followed by the treatment where the applied 5 mg L-1 of Pectimorf® (Figure 1).
These results demonstrate the promoting effect of Biobras-16® when applied exogenously, which coincides with other investigations that indicate its anti-stress activity at concentrations between 0.1 and 0.001 mg L-1 (7,8. This effect could be responsible for improving the germination percentage with the cryo-frozen treatments (four and eight months). On the other hand, it is mentioned that brassinosteroids positively affect fertilization, modifying the properties of pollen through the stimulation of its growth and pollen tubes. This has been attributed to its action on cell division and elongation 7. In studies carried out on the in vitro germination of pollen from Oryza sativa L 17) and on pollen from Arabidopsis thaliana18, the applying of 24-epibrasinolide improved the in vitro germination of pollen grains when they were subjected to heat stress conditions was shown.
Additionally, neither the brassinosteroid nor the highest concentration of oligogalacturonides had a significant effect on the area of the pollen grain was found; however, the concentration of 5 mg L-1 of Pectimorf® (M4) negatively affected this variable (Table 2).
Regarding to the pollen tube length of fresh pollen grains, it was evidenced that there are significant differences between some treatments (p=0.002). For this variable, the medium supplemented with 5 mg L-1 Pectimorf® (M4); showed greater elongation of the pollen tube (397±76), although it did not differ from the lower concentration of Biobras-16®, nor from the higher concentration of Pectimorf®. The treatments enriched with brassinosteroids (M2 and M3) and with 10mg L-1 of Pectimorf® (M5), did not show significant differences for this variable with respect to the control (Table 2, Figure 2).
Table 2 Effect of Biobras-16® and Pectimorf® on the area and length of the pollen tube of fresh teak pollen grains
| Germination media | Pollen grain area (mm2) p=0.007 E.S.x=323.4 | Pollen tube length (µm) p=0.002 E.S.x=81.3 |
|---|---|---|
| M1 | 1551.0 a | 199.9 b |
| M2 | 1354.0 a | 310.6 ab |
| M3 | 1269.1 ab | 150.6 b |
| M4 | 629.0 b | 397.2 a |
| M5 | 1334.0 a | 302.1 ab |
Means with a common letter are not significantly different according to the Tukey test (p> 0.05). M1: Without supplements, M2: 0.001 mg L-1 Biobras-16®, M3: 0.050 mg L-1 Biobras-16®, M4: 5 mg L-1 Pectimorf®, M5: 10 mg L-1 Pectimorf®
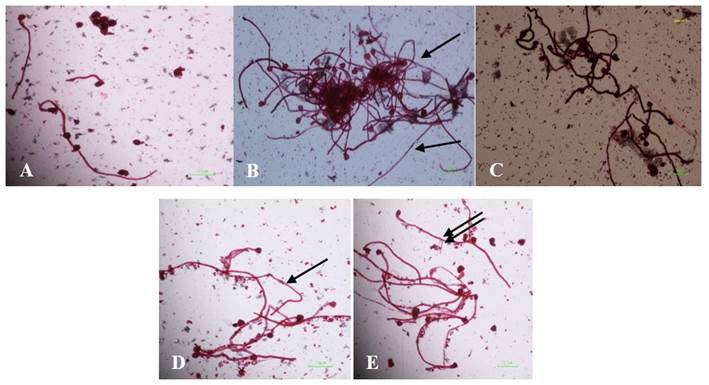
A: Without supplements, B: 0.001 mg L-1 Biobras-16®, C: 0.050 mg L-1 Biobras-16®, D: 5 mg L-1 Pectimorf®, E: 10 mg L-1 Pectimorf®
Figure 2 Images showing the effect of Biobras-16® and Pectimorf® on the pollen tube length of fresh teak pollen grains. The pollen tubes in the treatments that reached the greatest length are indicated
The results obtained agree with in vitro germination studies of pollen grains of Solanum tuberosum, in which Pectimorf® notably favored the growth of pollen tubes at a concentration of 5 mg L-1, noting a dependence on the genotype and the concentrations of the product in the response of plant material. It should be noted that this compound is known for its effect as a substitute for traditional growth regulators such as auxins and cytokinins, in in vitro culture at different stages and species 19.
The pollen tube is known to be composed of a rapidly growing cell that requires a massive deposition of the cell wall to promote its rapid elongation. The inner wall of the pollen tube is abundant in callose and contains a low amount of cellulose, while the outer layer is made mainly of pectins up, which, when deesterified and cross-linked by Ca2 +, provide rigidity to the cell wall, especially in the back of the tube 20
Due to this, it is worth mentioning that research carried out in Arabidopsis thaliana has shown that oligogalacturonides increase phosphorylation of phosphoproteins such as myosin 17 and the plasma membrane-associated cation-binding protein (PCaP1) 21. This could explain the positive effect of Pectimorf® on the elongation of the teak pollen tube, since myosin stimulates trafficking through actin of component-bearing vesicles for the synthesis of a new membrane and non-cellulosic cell wall towards the apical zone growth. Additionally, PcaP1 is capable of binding actin and calcium, which causes destabilization of actin filaments. The maintenance of the gradient of calcium ions in the cytosol allows the polarized growth of the pollen tube, for which the regulation of the entry of these ions through the plasma membrane is important 21,22.
Despite multiple reports that indicate that, brassinosteroids promote pollen tube growth by promoting cell elongation and division; this positive effect was not evidenced in fresh teak pollen. This could be because the response of plant species depends on the type and concentration of the brassinosteroid used, as well as the interaction of genetic, chemical and environmental factors, since the development of the pollen tube is very sensitive to stress factors 15.
When performing the correlation analysis between pollen size and elongation of the pollen tube, it was found that there is no linear correlation between the variables for the use of Biobras-16®, where the analysis showed randomness when evaluating its effect, in which a Pearson coefficient of 0.097 was obtained (Figure 3A). When analyzing the effect of Pectimorf®, a Pearson coefficient of -0.441 was obtained, so it was possible to affirm the existence of a negative correlation between the two variables for this product (Figure 3B).

A) Treatments supplemented with Biobras-16®, B) Treatments supplemented with Pectimorf®
Figure 3 Correlation analysis between the variables pollen grain area and length of pollen tube
The existence of a negative correlation between the variables pollen grain area and pollen tube length in the treatments supplemented with Pectimorf®, suggests a tendency for smaller pollen grains to have a greater ability to grow on the pistil and the possibility to be more effective in fertilizing stigma 19. This behavior possibly responds to the fact that oligogalacturonides are chemical messengers that perform functions similar to auxins, modulate the enzymes involved in specific processes in the plant, such as morphogenesis, germination and growth 11. When pollen germination begins, there is a displacement of the cytoplasm towards the apical region of elongation and as the pollen tube elongates, callose and transporter vesicles are deposited in said region, attending to the polarized growth that characterizes it 23,24. These results coincide with the trends observed in the in vitro germination of eggplant (Solanum melongena) pollen without bioactive supplements 25.
CONCLUSIONS
The bioactive substances evaluated do not favor the germination of fresh teak pollen, but Biobras-16®, in a concentration of 0.001 mg L-1, stimulates the germination of cryopreserved teak pollen for four and eight months. Pectimorf® favors the growth of the pollen tube of fresh samples of teak pollen, mainly in the concentration of 5 mg L-1.
A negative correlation was evidenced for the length of the pollen tube and the pollen area when supplementing the germination culture medium with this product.
RECOMMENDATIONS
It is recommended to evaluate the viability of pollen in longer periods of cryopreservation and to use different concentrations of these bioactive compounds to carry out predictive models.
In addition, it is advisable to analyze the variables pollen tube length and pollen area with the largest number of samples to determine more accurately correlations between these factors, as well as to study these variables in cryopreserved pollen.
ACKNOWLEDGMENT
This research had the financial support of the Vice-rector for Research of the National University of Costa Rica, as well as the Institute of Forestry Research and Services of the UNA, the Vice-rectory for Research and Extension of the Technological Institute of Costa Rica, of the improvement cooperative forest genetics GENFORES. Besides, from the Ministry of Science, Technology and Telecommunications of Costa Rica (MICITT) as well as the special support of the company Novalteak Costa Rica S.A. Also, thank the National Institute of Agricultural Sciences (INCA) for the contribution of these new substances used in research.
REFERENCES
1. Pooja V, Dinesh KY, Poonam K. Tectona grandis (teak): A review on its phytochemical and therapeutic potential. Natural Product Research. 2019; 33(16):2338-2354. [ Links ]
2. Yasodha R, Vasudeva R, Balakrishnan S, Sakthi AR, Abel N, Binai N, et al. Draft genome of a high value tropical timber tree, Teak (Tectona grandis L. f.): insights into SSR diversity, phylogeny and conservation. DNA Research. 2018;25(4):409-19. [ Links ]
3. Jalca I, García L, Castro O, Villamar R, Guachambala M. Condiciones óptimas para almacenamiento del polen de Ochroma pyramidale. Bosque (Valdivia). 2019;40(2):227-33. [ Links ]
4. Martínez-Pacheco J. In vitro germination of tobacco (Nicotiana tabacum L.) pollen. Cultivos Tropicales. 2018;39(1):102-7. [ Links ]
5. Rodríguez TJ, Rodríguez MA, Canul-Ku J, Castillo A, Martínez E, Guillén D. Viabilidad de polen, receptividad del estigma y tipo de polinización en cinco especies Echeveria en condiciones de invernadero. Revista Mexicana de Ciencias Agrícolas. 2015;6(1):111-23. [ Links ]
6. Ali B. Brassinosteroids: The Promising Plant Growth Regulators in Horticulture. In: Hayat et al. editors. Brassinosteroids: Plant Growth and Development. Springer Nature Singapore Pte Ltd; 2019. p. 349-365. doi: 10.1007/978-981-13-6058-9_12. [ Links ]
7. Izquierdo H. Actividad biológica de los brasinoesteroides y sus análogos en las plantas. Temas de Ciencia y Tecnología. 2011;15(43):45-50. [ Links ]
8. Martínez-González L, Reyes-Guerrero Y, Pérez-Domínguez G, García M, Núñez-Vázquez M, et al. Influencia del Biobras-16(r) y el QuitoMax(r) en aspectos de la biología de plantas de frijol. Cultivos Tropicales. 2018;39(1):108-12. [ Links ]
9. Lara D, Costales D, Falcón A. Los oligogalacturónidos en el crecimiento y desarrollo de las plantas. Cultivos Tropicales. 2018;39(2):127-34. [ Links ]
10. Posada-Pérez L, Padrón-Montesinos Y, González-Olmedo J, Rodríguez-Sánchez R, Barbón-Rodriguez R, et al. Efecto del Pectimorf(r) en el enraizamiento y la aclimatización in vitro de brotes de papaya (Carica papaya L.) cultivar Maradol Roja. Cultivos Tropicales. 2016;37(3):50-9. [ Links ]
11. Ramos L, Arozarena NJ, Lescaille J, García F, Tamayo Y, Castañeda E, et al. Dosis de Pectimorf(r) para enraizamiento de esquejes de guayaba var. Enana Roja Cubana. Revista Mexicana de Ciencias Agrícolas. 2013;6:1093-1105. [ Links ]
12. Hine A, Rojas A, Murillo O. Effect of liquid nitrogen on the viability and germination of teak pollen. In: 5th International Conference of the IUFRO Working Party 2-09.02 Somatic embryogenesis and Other Vegetative Propagation Technologies. Coimbra, Portugal; 2018. p 112. [ Links ]
13. Brewbaker J, Kwack B. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany. 1963;50(9): 859-65. DOI: 10.2307/2439772 [ Links ]
14. Hine A, Rojas A, Suarez L, Murillo O, Espinoza M. Optimization of pollen Germination in Tectona grandis (Teak) for Breeding programs. Forests. 2019;10(908):1-10. [ Links ]
15. Hernández E, García I. Brasinoesteroides en la agricultura II. Revista Mexicana de Ciencias Agrícolas. 2016;7(2):451-62. [ Links ]
16. Jia MX, Shi Y, Di W, Jiang XR, Xu J, et al. ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. In Vitro. Cell.Dev.Biol.-Plant. 2017;53:433-39. [ Links ]
17. Thussagunpanit J, Jutamanee K, Kaveeta L, Chai-arree W, Pankean P, Suksamrarn A. Effects of a brassinosteroid and an ecdysone analogue on pollen germination of rice under heat stress. Journal of Pesticide Science. 2013;38(3):105-11. [ Links ]
18. Vogler F, Schmalzl C, Englhart M, Bircheneder M, Sprunck S. Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reproduction. 2014;27(3):153-67. doi: 10.1007/s00497-014-0247-x. [ Links ]
19. Suárez L, Hernández M. Efecto de Pectimorf en la germinación in vitro del polen de papa (Solanum tuberosum). Temas de Ciencia y Tecnología. 2010; 14(40):43-6. [ Links ]
20. Wang L, Wang W, Wang YQ, Liu YY, Wang JX, et al. Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol Plant. 2013;6(4):1131-48. doi:10.1093/mp/sst084 [ Links ]
21. Mattei B, Spinelli F, Pontiggia D, De Lorenzo G. Comprehensive Analysis of the Membrane Phosphoproteome Regulated by Oligogalacturonides in Arabidopsis thaliana. Front. Plant Sci. 2016;7:1107. doi:10.3389/fpls.2016.01107 [ Links ]
22. Michard E, Simon A, Tavares B, Wudick M, Feijó JA. Signaling with Ions: The Keystone for Apical Cell Growth and Morphogenesis in Pollen Tubes. Plant Phys. 2017;173(1):91-111. doi: 10.1104/pp.16.0156. [ Links ]
23. Cheung AY, Wu H. Structural and Signaling Networks for the Polar Cell Growth Machinery in Pollen Tubes. Annu Rev Plant Biol. 2008;59:547-72. [ Links ]
24. Zhang R, Qu X, Zhang M, Jiang Y, Dai A, et al. The Balance between Actin-Bundling Factors Controls Actin Architecture in Pollen Tubes. iScience. 2019;16:162-176. doi:10.1016/j.isci.2019.05.026 [ Links ]
25. Araméndiz H, Cardona C, Lugo E. Germinación del polen de berenjena (Solanum melongena L.) en condiciones in vitro. Rev. Fac. Nac. Agron. Medellin. 2012;65(2):6643-49. [ Links ]
Received: May 16, 2019; Accepted: September 07, 2020











 text in
text in 



