My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Cultivos Tropicales
Print version ISSN 0258-5936On-line version ISSN 1819-4087
cultrop vol.42 no.1 La Habana Ene.-Mar. 2021 Epub Mar 31, 2021
Original article
Acclimatization of Lisianthus (Eustoma grandiflorum (Raf.) Shinners) seedlings ‘Mariachi blue’ cultivar with an oligogalacturonide
1Universidad Agraria de La Habana “Fructuoso Rodríguez Pérez”, carretera a Tapaste y Autopista Nacional, San José de las Lajas, Mayabeque, Cuba
2Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
3Centro de Investigación en Química Aplicada (CIQA). Saltillo, Coahuila de Zaragoza. México
4Unidad Docente “William Soler” de la Universidad Agraria de La Habana (UNAH), carretera Bejucal Quivicán, Quivicán, Mayabeque, Cuba. CP 33 500
Oligogalacturonides are substances that regulate the growth and plant development and stimulate defensive responses; however, there is no information on the effects that these compounds exert when in vitro seedlings of Lisianthus (Eustoma grandiflorum (Raf.) Shinners) are transferred to ex vitro conditions. Therefore, an experiment was carried out with the objective of evaluating the oligogalacturonide influence of pectic origin (mOLG) in Lisianthus seedlings of cultivar ‘Mariachi blue’ during the first acclimatization phase. The treatments consisted on seedling foliar spraying at transplantation time and 15 days after it, at a rate of 2 mgL-1 of IAA; 1, 5 and 10 mgL-1 of mOLG and a control treatment was used in which no foliar spraying was performed. Morphological variables were evaluated: height and number of leaves per plants and physiological variables: fresh and plant dry mass, relative water content (RWC) and photosynthesis. The experiment was carried out in a completely randomized design with three replications. The data were processed using a simple ANOVA and the means between the treatments were processed using the Tukey test (p(0.05). Plants sprayed with mOLG (1 mgL-1), reached the highest height [9.37 cm], the number of leaves ranged between 9-10; and those that were treated with mOLG (10 mgL-1), presented higher fresh mass; However, the plants treated with mOLG (1 mgL-1), reached higher dry mass (0.209845 g), CRA (90.75 %) and photosynthesis (12.873 μmol CO2 m-2s-1). There is potential for the cultivation of this cut flower in Cuba.
Key words: adaptation; growth bioestimulator; physiology; flowers; survival
INTRODUCTION
Lisianthus (Eustoma grandiflorum (Raf.) Shinners), belongs to the Gentianaceae family, is a biennial herbaceous plant, grown as an annual, with an erect stem, with showy foliage and flowers 1. It is an ornamental cut plant, native to the states of northern Mexico and southern United States. Currently, it is grown mainly in Japan, Europe and the United States due to the great diversity of flowers and high productivity it presents 2. Its natural habitat allows it to adapt to conditions of low relative humidity and temperatures, to some extent, extreme; it grows along the river and stream beds, where it always has access to water 3.
The interest in this species production is related to flower great diversity and their high productivity 4. Lisianthus is a relatively new flower crop on the international market, yet it has quickly become one of the top cut flowers globally. In 2012 in the United States and Taiwan between 10 and 14.9 million stalks of Lisianthus were marketed, respectively. In 2008, 23.4 million stems of this species were marketed in Japan. Furthermore, it should be noted that Lisianthus ranked third among the ornamental species by planted area (435 ha) and in that same year, in Taiwan it was ranked sixth also by planted area (130 ha), while in Netherlands reached the tenth place (45 ha). During the years 2017 and 2018, the sowing area of this ornamental species has continued to increase due to its high productive potential and for its commercialization 5.
According to studies carried out 6, the propagation by Lisianthus seeds is complicated and difficult due to slow germination and growth. Furthermore, the seedling populations that are obtained are variable with respect to flowering time, stem length and flower qualities. Vegetative propagation of selected cultivars can provide a useful alternative to seed propagation.
Therefore, micropropagation serves as a powerful tool to develop a rapid propagation method of selected Lisianthus genotypes. Clonal multiplication of these cut flowers, especially through tissue culture, can provide a useful alternative to seed propagation, resulting in higher quality planting material. The in vitro multiplication of elite genotypes offers the opportunity to multiply a disease-free large quantity and vigorous planting material in the shortest possible time 6.
The introduction in Cuba, through the exchange of germplasm is of great importance, since it contributes to increase the diversity of cut flowers, once a methodology for their multiplication is in place. On the other hand, new horizons would be opened for research in physiology and genetic improvement for the edaphoclimatic conditions of the country.
Once the seedling in vitro propagation is completed, it is essential to adapt them to uncontrolled ex vitro environmental conditions, both in the grow house and in field conditions. This phase is known as acclimatization. Plants must adapt from the morphological and physiological point of view after their transfer from in vitro culture to ex vitro conditions, that is, they change their heterotrophic or mixotrophic metabolism to autotrophic 7.
As a result of the in vitro environment, plants present an anatomy and physiology different from those that are cultivated in field conditions or cultivation houses 8. The observed disorders affect all plant organs, although not all have the same weight on the ex vitro behavior. Among these disorders are the poor development of the photosynthetic apparatus, of leaf cuticle, the emission of non-functional roots without connection with the conductive bundles, and others that can affect plant survival in the acclimatization phase 9.
The active substance introduction of national production in the in vitro Lisianthus plant regeneration methodology, could constitute an alternative to improve the rooting and ex vitro seedling acclimatization.
One of the most widely used bioactive substances today is the nationally produced growth biostimulator called Pectimorf®, which is a mixture of (1-4) α-D-oligogalacturonides, with a polymerization degree between 9 and 16 10,11. It has been studied in crops such as papaya (Carica papaya L.) cultivar 'Maradol roja' (16) and garlic (Allium sativum L.) clone 'Criollo-9' (17) with favorable results. It would be an alternative for the in vitro propagation of different Lisianthus cultivars, which would achieve greater uniformity of seedlings and achieve greater vigor and survival of them during the acclimatization phase.
For all the above, the objective of this work was to evaluate the oligogalacturonide influence of peptic origin (mOLG) on Lisianthus cultivar ‘Mariachi blue’ vitroplants during the acclimatization phase.
MATERIALS AND METHODS
The experiment was carried out in Genetics and Plant Improvement Department of the National Institute of Agricultural Sciences, Cuba.
Vegetal material
Vitroplants of Lisianthus (Eustoma grandiflorum (Raf.) Shinners) cultivar 'Mariachi blue' of at least 3 cm in height and 4 leaves, from the in vitro multiplication phase, obtained in a culture medium DM+6-BAP (2 mgL-1) were used and without roots, from the third subculture, which were carried out between 21-25 days and all the in vitro seedlings came from the same micropropagation lot.
Growing conditions
The vitroplants from the in vitro culture were planted in plastic trays of 40 alveoli, which had a capacity of 90.0 cm3 and contained a substrate composed of organic matter (filter cake) and compacted Eutric Red Ferrallitic soil, in a volume ratio of 75 and 25 %, respectively. The substrate used was not disinfected. The seedlings were guaranteed watering by nebulization in the first 7 days, for 2 minutes (9:00 am, 12:00 pm and 3:00 pm) to achieve high relative humidity (90-95 %). For shading a black mesh was used (70 % reduction of sunlight). Substrate chemical characteristics are shown in Table 1.
Table 1 Substrate chemical characteristics used for the Lisianthus (Eustoma grandiflorum (Raf.) Shinners) vitroplant acclimatization cultivar ‘Mariachi blue’
| Substrate | pH H2O | M.O (%) | P (ppm) | K | Ca | Mg | Na |
|---|---|---|---|---|---|---|---|
| Cmolkg-1 | |||||||
| Filter cake: Soil (75 %: 25 %) | 7,0 | 13,93 | 2640 | 4,71 | 29,4 | 8,27 | 0,49 |
The treatments were carried out by foliar spraying of the seedlings at the time of transplantation to the plastic trays and 15 days after transplantation they were sprayed at a rate of 2 mL per seedling.
Treatments
Control (without foliar spraying).
Foliar spray at the time of transplantation and 15 days after transplantation with IAA (2 mgL-1).
Foliar spray at the time of transplantation and 15 days after transplantation with mOLG (1 mgL-1).
Foliar spray at the time of transplantation and 15 days after transplantation with mOLG (5 mg L-1).
Foliar spray at the time of transplantation and 15 days after transplantation with mOLG (10 mgL-1).
Evaluations
After 7 days, the different evaluations were carried out and at 60 days the data were taken during the acclimatization phase. It was evaluated:
Plant survival percentage: Total of living explants that survived with respect to the total of explants, to later determine the survival percentage.
Plant height (cm): It was measured with a graduated ruler, from substrate base to the last fully extended leaf.
Plant rooting percentage: Total of rooted plants with respect to the total that was transplanted and later rooting percentage was determined.
Number of leaves per plants: The total number of leaves was counted and the means for each treatment were subsequently obtained.
In the case of the physiological variables, three plants were taken at random from the different treatments and it was determined:
Plant fresh mass (PFM) (g): The mass of the stem and leaves, as well as the roots (in the plants that presented this organ) of the plants was determined in an analytical balance brand SARTORIUS (France). (0,001 g
Plant dry mass (PDM) (g): Plants were taken, which were placed in a MEMMERT brand oven (Germany) at a temperature of 75 ± 5 °C for 72 hours and subsequently, the mass was determined on a balance SARTORIUS (France) brand analysis until the mass was constant.
Total chlorophyll content (TCC) (SPAC Units): Five leaves of each plant were used. Measurements were made in SPAC units and a Minolta SAPD-502 Plus Chlorophyll Meter manufactured in Spain was used.
Relative Water Content (RWC) [%]: It was determined in the first two leaves of the plant (youngest leaves), measured from the apex towards the base of the same and with an instrument of 0.5 cm in diameter, Between 4-5 discs were extracted from the leaf, the mass of which was determined and they were placed in distilled water for 4 h. Subsequently, the mass was determined again and they were placed in a MEMMERT brand oven (Germany) at a temperature of 75 ± 5 °C for 72 hours and subsequently, the mass was determined on a SARTORIUS brand analytical balance (France) until the mass was constant and the RWC was calculated with the formula:
RWC = [(fresh mass-dry mass)/( fresh mass-dry mass)] x 100
Maximum photosynthesis (μmol CO2 m-2s-1): For the determinations, fully expanded leaves were used in the same position (leaves one and two) counted from the base towards the apex, at 60 days. The determinations were made in three plants by treatments with five measurements in each one for a total of 15 measurements. It was determined with the CIRAS-2 equipment (Portable Photosynthesis System, United Kingdom) coupled to a PLC6 universal tray. Measurements were always made between 8:30 -9: 30 am.
Total perspiration (mmol H2O m-2 s-1): A similar procedure was followed to determine the maximum photosynthesis.
Stomatal conductance (CE) (mmol m-2s-1): A similar procedure was followed to determine maximum photosynthesis.
Experimental design and data analysis
A completely randomized design with 10 plants per treatment was used. The experiment was repeated three times. The data were processed by Simple Classification Analysis of Variance (ANOVA) with the SPSS 11.5 program for Windows (SPSS, Inc., Chicago, IL) and the comparison between the means was made according to the Tukey test (p(0,05). In all cases, the normal distribution (Kolmogorov-Smirnov) and the homogeneity of variance (Bartlett) 12 were previously checked.
RESULTS AND DISCUSSION
In relation to the percentage of survival of the plants, there were significant differences between the treatments (Figure 1). The best results were obtained by the plants of treatment 3 [Foliar spray at the time of transplantation (AFMT) and 15 days after transplantation (AFDT) with mOLG (1 mgL-1)], with 100 % survival and it differed from the rest of treatments. In general, the plants when the IAA or the mOLG was applied regardless of the concentration, the survival was high, it ranged between 90-100 %; only the plants of the control treatment obtained a survival of less than 90 %.
According to some authors 13, when transferring Lisianthus seedlings from in vitro culture to a substrate composed of a mixture of burnt rice husk and organic manure, and immersing them in a 1 % pesticide solution and covering the pots of transparent plastic for 15 days in an area of reduced light intensity (100-120 μmolm-2s-1) produced a high seedling survival (81-100 %), 90 % on average after one acclimatization month.
In studies carried out on Lisianthus seedlings 14, in a basal culture medium DM enriched with AIB (0.5 and 1.5 mgL-1) they presented a better percentage of rooting in vitro; they acclimatized better and when transferred to the greenhouse they showed normal growth and survival of 90 %.
Figure 1 shows the percentage of rooting of the plants, and there were significant differences between the treatments. The best results were obtained with the use of IAA (Treatment 2) and mOLG (1, 5 and 10 mgL-1) with AFAT and with AFDT, with 100 % rooting, without differences between them, but with the treatment control, which obtained 90 % rooting.
The best results with Lisianthus cultivar 'Azul' plants, according to some authors 15, were obtained with IAA concentrations of 1000 and 500 ppm (mgL-1), equivalent to 100 and 92 % rooting, respectively.
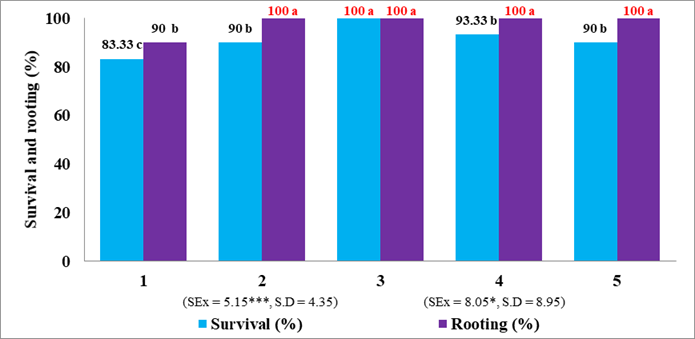
Means with different letters differ statistically according to Tukey's test (p(0.05) (* significant for p <0.1; *** significant for p(0.001)
1- Control (without foliar spraying)
2- Foliar spray at transplantation time and 15 days after transplantation with IAA (2 mg L-1)
3- Foliar spray at transplantation time and 15 days after transplantation with mOLG (1 mg L-1)
4- Foliar spray at transplantation time and 15 days after transplantation with mOLG (5 mg L-1)
5- Foliar spray at transplantation time and 15 days after transplantation with mOLG (10 mg L-1)
SEx.- standard error of the mean. S.D.- standard deviation
n- Total of explants of the experiment in the three repetitions
IAA-indol-acet -acid
mOLG.- mixture of oligogalacturonides
Figure 1 Rooting and survival percentage of Lisianthus plants (Eustoma grandiflorum (Raf.) Shinners) cultivar 'Mariachi blue' treated or not with Pectimorf® mOLG, at 60 days after the end of the acclimatization phase (n=30)
On the other hand, some authors 16 stated that in papaya (Carica papaya L.) cultivating 'Maradol roja', the highest percentage of plants with roots (84.2 %) was obtained with a concentration of 9 mgL-1 of Pectimorf® in complement with auxin AIB 16.
Also other authors reported in garlic (Allium sativum L.) clone 'Criollo-9', that the rooting of the microbulbs was increased by immersion 15 minutes and foliar spraying 15 days after planting in a Pectimorf® solution (10 mgL-1) and when they were planted in a substrate composed of zeolite [lithonite] (25 %) and organic matter [decomposed filter cake] (75 %) 17.
There may be an oligogalacturonide biosynthesis activation as mOLG when they are applied exogenously in plants and influence their survival, and consequently, their growth and development. On the other hand, during the acclimatization phase, it is likely that the plants accumulated greater biomass during the in vitro phase, which favorably conditioned them to survive the stress caused by the change to ex vitro conditions.
Regarding plant height, there were significant differences between treatments (Figure 2). The best results were obtained by treatment 3 seedlings that were treated with mOLG (1 mgL-1) [9.37 cm] and the lowest were those of treatment 1 (Control) [6.53].
Lisianthus plants, treated with different doses of nitrogen (50-300 mg) supplied by fertigation, had no influence on peduncle height, which ranged between 3.31-5.06 cm 18.
According to some authors, they reported that the best results were obtained with the immersion of the roots of the seedlings for 15 minutes and the foliar spraying of them 15 days after planting with Pectimorf® (5 and 10 mgL-1), whose respective values were 14.17 and 14.42 cm 19, without statistical differences between them or with ANA at a concentration of 10 mgL-1, with 14.12 cm in height.
In Figure 2, it can be seen that there were no significant differences between the treatments with respect to the number of leaves, which ranged 9-10 per plants. Similar results reported in garlic clone 'Criollo-9' 17 with a number of leaves was 2.37 when the roots were immersed for 15 minutes in a Pectimorf® solution (10 mgL-1), but without statistical differences with this oligosaccharin, at the concentration of 5 mgL-1 (2.27) or in ANA (10 mgL-1) [2.20] and the foliar spraying of the seedlings 15 days after planting with the same concentration of those substances.

Means with different letters differ statistically according to Tukey's test (p(0.05) (*** significant for p(0.001)
1- Control (without foliar spraying)
2- Foliar spray at transplantation time and 15 days after transplantation with IAA (2 mg L-1)
3- Foliar spray at transplantation time and 15 days after transplantation with mOLG (1 mg L-1)
4- Foliar spray at transplantation time and 15 days after transplantation with mOLG (5 mg L-1)
5- Foliar spray at transplantation time and 15 days after transplantation with mOLG (10 mg L-1)
SEx.- standard error of the mean. S.D.- standard deviation
n- Total of explants of the experiment in the three repetitions
IAA- Indol acetic acid
mOLG.- mixture of oligogalacturonides
Figure 2 Height (cm) and Leaf number of Lisianthus plants (Eustoma grandiflorum (Raf.) Shinners) cultivar 'Mariachi blue' treated or not with mOLG, a 60 days after the acclimatization phase (n = 30)
In relation to the fresh and plant dry mass, there were significant differences between the treatments (Table 2). In the first case, the best results were for the plants of treatment 5 (1.88888) and in the second case, those of treatment 3 (0.209845). In both cases, the seedlings treated with mOLG regardless of the concentration (1, 5 or 10 mgL-1) achieved superior results than those treated with IAA or those of control treatment.
According some authors, to Lisianthus seedlings cultivar ‘Mariachi blue’, cultivated in modified zeolite loaded with Ca2+ showed a seedling higher fresh mass. The raised by the previous author, it may be due to the fact that calcium is involved in cell division, permeability of cell membranes, thus providing vigor to the plant 2.
Some authors, reported that in the potato (Solanum tuberosum L.) crop the fresh mass of the minitubers was higher in the 'Yuya' varieties with 18.67 g and 'Grettel' with 23.20 g without differences significant among them and yes with the rest of the varieties (20). Similarly, 'Grettel' showed the highest values in the minituber dry mass with 19.3 g without significant differences with 'Ibis' which reached 19.4 g.
According to studies carried out, the variables fresh mass and dry mass of the studies carried out 21, the variables fresh mass and dry mass of the rhizomes did not show differences between the evaluated treatments, obtaining values of 11.88 g and 2.52 g respectively, similar values to those obtained by the culture.
Table 2 Fresh and dry mass, as well as total chlorophyll content, relative water content, maximum photosynthesis, total transpiration and stomatal conductance of Lisianthus plants (Eustoma grandiflorum (Raf.) Shinners) cultivar 'Mariachi blue' treated or not with the mOLG, 60 days after acclimatization phase end (n = 5)
| Nu. | PFM (g) | PDM (g) | TCC (Units SPAC) | RWC (%) | Photosynthesis (μmol CO2 m-2s-1) | Transpiration (mmol H2O m-2s-1) | SC (mmolm-2s-1) |
|---|---|---|---|---|---|---|---|
| T1 | 1.58 e | 0.15 e | 56.33 d | 80.00 c | 10.81 d | 9.00 e | 0.33 d |
| T2 | 1.61 d | 0.16 d | 60.08 c | 81.85 b | 11.54 c | 9.47 d | 0.40 c |
| T3 | 1.70 c | 0.20 a | 70.97 a | 90.75 a | 12.87 a | 11.27 a | 0.50 a |
| T4 | 1.75 b | 0.17 c | 66.66 b | 89.57 a | 12.12 b | 10.84 b | 0.46 b |
| T5 | 1.88 a | 0.18 b | 62.01 c | 82.12 b | 12.09 b | 10.00 c | 0.40 c |
| S.Ex (() | 0.0052*** | 0.00061** | 0.27*** | 1.14* | 0.39** | 0.34*** | 0.04*** |
| S.D | 0.07 | 0.01 | 3.32 | 1.80 | 0.45 | 0.41 | 0.05 |
PFM-Plant fresh mass, PDM-Plant dry mass, TCC-Total chlorophyll content, RWC-Relative water content, SC-Stomatal conductance, SEx-Standard error of the mean, SD- Standard deviation
IAA- Indol-acetic acid, mOLG-oligogalacturonides mixture
n- Total of explants of the experiment in the three repetitions.
Means with different letters differ statistically according to Tukey's test (p(0.05) (*** significant for p(0.001)
1- Control (without foliar spraying)
2- Foliar spray at transplantation time and 15 days after transplantation with IAA (2 mg L-1)
3- Foliar spray at transplantation time and 15 days after transplantation with mOLG (1 mg L-1)
4- Foliar spray at transplantation time and 15 days after transplantation with mOLG (5 mg L-1)
5- Foliar spray at transplantation time and 15 days after transplantation with mOLG (10 mg L-1).
In the chlorophyll content in the plants there were significant differences between the treatments, as reflected in Table 2. The best results were achieved by the plants of treatment 3 (AFMT) and (AFDT), with 70.97 SPAC units, which were differentiated from the plants of the control treatment, with 56.33 SPAC units. In general, the seedlings that were treated with mOLG regardless of the concentration were superior to those of treatment rest, once the acclimatization phase had finished.
The previous results could indicate that the plants treated with mOLG (Pectimorf®) at the end of their acclimatization, are better nourished and it is possible that they have a higher content of total chlorophylls and, therefore, are ready for their definitive transplantation to pots or field.
According to some research carried out, with different hybrid Lisianthus lines, a high chlorophyll percentage (SPAC units) was found in lines L5 ('Nandini Lemon Doublel') [60.5 %], followed by L9 ('Nandini Royal Violoet') [58.3%] and L8 ('Nandini rose') [54.8 %], while the worst results were for the hybrid L15 ('Nandini Lavender') [33.3 %] 22. Other authors also observed a similar variation in chlorophyll percentage in a study carried out with eight lines of Lisianthus 23; as well as, in chrysanthemum 24.
According to studies carried out, they reported that Lisianthus seedlings presented a similar response in terms of pigment content by increasing Electrical Conductivity (EC) [2.5; 4; 6 and 8 dSm-1] and the respective total chlorophyll content was: 2.85; 3.08; 2.05 and 2.79 mg g-1 (25. They added that in the 6 dSm-1 solutions a decrease in chlorophyll concentration was observed, which was later recovered when the plants were irrigated with 8 dSm-1 solutions; and plants treated with additional Ca2+ managed to maintain a higher concentration of pigments compared to those treated with 9 meqL-1 of Ca2+ when the EC of the solution was 6 and 8 dSm-1.
The results related to the Relative Water Content (RWC) are shown in Table 2. There were significant differences between the plants of the different treatments. The best results were for treatments 3 and 4, with 90.75 and 89.57 %, respectively, without significant differences between them; while the lowest results were for the plants that were obtained with treatment 1 (control), with 80.00 %.
In studies carried out with Lisianthus plants 25, the RWC in young leaves ranged between 69.7-89.1 % and in mature ones (76.0-80.3 %); in both cases, in general, it decreased in plants with 9 meqL-1 of Ca2+; however, in plants supplemented with additional Ca2+, an increase in RWC was observed, which exceeds that of plants with 9 meqL-1 when treated with solutions of CE greater than 4 dSm-1.
RWC maintenance in young leaves was related to the accumulation of a higher biomass by the plants treated with mOLG, which could also be related to a greater water potential in the leaves.
The growth biostimulators Biobras-6® (0.2 (molL-1) and Pectimorf® (0.47 (molL-1), positively influenced banana plants RWC (Musa spp.) Clone 'FHIA-18 '(AAAB), when their roots were submerged and 15 days after planting they were sprayed with the previous solutions, the values were 96.64 and 96.7 %, respectively 26.
Photosynthesis, transpiration and stomatal conductance were other physiological variables that were evaluated at the end of the acclimatization phase of the Lisianthus cultivar ‘Mariachi blue’ plants. There were significant differences between the treatments (Table 2). The best results were obtained by the plants of treatment 3 (AFMT and AFDT), with a photosynthesis of 12,873 μmol CO2m-2s-1, transpiration was 11.273 mmol H2Om-2s-1 and stomatal conductance of 0.508 mmolm-2s-1. In a general sense, the plants obtained with mOLG (Pectimorf®), regardless of the concentration, showed the best results, when the previous physiological variables were evaluated.
Other authors stated that the plants of the Lisianthus cultivar 'ABC Blue', with regardless of the concentration of Ca2+ in the nutrient solution, the EC affected the photosynthesis rate by presenting a decrease 25) when the EC was 4 dSm -1 (with 9 meqL-1 of Ca2+, photosynthesis was 10.1 µmol CO2m-2s-1 and with 13 meqL-1 of Ca2+, photosynthesis was 9.4 µmol CO2m-2s-1). However, when it was increased to 6 dSm-1 (with 9 meqL-1 of Ca2+, photosynthesis was 10.6 μmol CO2m-2s-1 and with 13 meqL-1 of Ca2+, photosynthesis was 11.4 μmol CO2m -2s-1) and 8 dSm-1 (with 9 meqL-1 of Ca2+, photosynthesis was 12.4 μmol CO2m-2s-1 and with 13 meqL-1 of Ca2+, photosynthesis was 12.0 μmol CO2m-2s-1), the photosynthesis rate recovers to levels comparable to that of plants with low EC.
In general, in Lisianthus plants cultivating 'ABC Blue', leaf conductance (with 9 meqL-1 of Ca2+, ranged between 0.241-0.308 mol H2Om-2s-1 and with 13 meqL-1 of Ca2+, it was between 0.219-0.312 mol H2Om-2s-1), as well as the transpiration rate (with 9 meqL-1 of Ca2+, it ranged between 9.2-10.2 mmol H2Om-2s-1 and with 13 meqL-1 of Ca2+, it was between 8.6-10.5 mmol H2Om-2s-1), generally decreased as the EC was increased, regardless of the level of Ca2 + in the nutrient solution 25.
The results obtained in the in vitro papaya plants (Carica papaya L.) cultivating 'Maradol roja' at 37 days of culture, during the acclimatization phase, showed that using AIB (2 mgL-1) with zeolite as a support, they achieved the best results with respect to photosynthesis (3.828 μmol CO2m-2s-1), perspiration (1.506 mmol H2Om-2s-1) and stomatal conductance (34.85 mmolm-2s-1) and surpassed the treatments in which Pectimorf® (7, 9 and 12 mgL-1) and AIB (2 mgL-1) were used 17.
The results of the previous authors 17 do not coincide with those of this work, since the mOLG (Pectimorf®) regardless of the concentration used (1, 5 and 10 mgL-1) enhance photosynthesis, transpiration and stomatal conductance of Lisianthus plants cultivar 'Mariachi blue'. Therefore, seedling acclimatization, a substrate composed of 75 % organic matter (decomposed filter cake) and 25 % of compacted Eutric Red Ferrallitic soil must be used and the seedlings foliar spraying at transplantation time and 15 days after planting at a rate of 2 mL per seedling with mOLG (Pectimorf® [1 mgL-1]).
BIBLIOGRAFÍA
1. Hernández C. Respuesta de lisianthus (Eustoma grandiflorum [Raf.] [Internet]. Colegio de Postgraduados; 2011 [cited 18/01/2021]. Available from: https://go.gale.com/ps/i.do?p=IFME&sw=w&issn=&v=2.1&it=r&id=GALE%7CA568727276&sid=googleScholar&linkaccess=abs [ Links ]
2. Enríquez, G. Germinación y producción de plántula de Lisianthus (Eustoma grandiflorum (Raf.) Shinners.) var. 'Mariachi blue', en mezclas de Peat-Moss y Zeolita. [Tesis de Ingeniería]. [Tenancingo, Estado de México]: Universidad Autónoma del Estado de México; 2017. 67 p. [ Links ]
3. de La Riva-Morales FP, Mazuela-Águila PC, Urrestarazu-Gavilán M. Comportamiento productivo del lisianthus Eustoma grandiflorum [Raf.] Shinn) en cultivo sin suelo. Revista Chapingo. Serie horticultura. 2013;19(2):141-50. [ Links ]
4. Barbaro LA, Karlanian MA, Morisigue D. El sistema flotante como alternativa para la producción de plantines de Lisianthus Eustoma grandiflorum L.). Agriscientia. 2009;26(2). [ Links ]
5. Fernández-Pavía YL, Trejo-Téllez LI. Biología, importancia económica y principales líneas de investigación en lisianthus: una especie ornamental nativa de méxico. AGROProductividad. 2018;11(8). [ Links ]
6. Nomita L, Raj K, Arvinder S. Lisianthus micro propagation. International Journal of Agricultural Sciences. 2012;8(2):541-6. [ Links ]
7. Martins JPR, Verdoodt V, Pasqual M, De Proft M. Impacts of photoautotrophic and photomixotrophic conditions on in vitro propagated Billbergia zebrina Bromeliaceae. Plant Cell, Tissue and Organ Culture (PCTOC). 2015;123(1):121-32. [ Links ]
8. Gutiérrez-Mora A, González-Gutiérrez AG, Rodríguez-Garay B, Ascencio-Cabral A, Li-Wei L. Plant somatic embryogenesis: some useful considerations. Embryogenesis. 2012;229-48. [ Links ]
9. Alvarez C, Sáez P, Sáez K, Sánchez-Olate M, Ríos D. Effects of light and ventilation on physiological parameters during in vitro acclimatization of Gevuina avellana mol. Plant Cell, Tissue and Organ Culture (PCTOC). 2012;110(1):93-101. [ Links ]
10. Cabrera JC, Gómez R, Diosdado E, Hormaza JV, Iglesias R, Gutiérrez A, et al. Procedimiento de obtención de una mezcla de oligosacáridos pécticos estimuladora del enraizamiento vegetal. Patente Cubana. 2003;22859. [ Links ]
11. Falcón R, Costales M, González-Peña F, Nápoles G. New natural products for agriculture: the oligosaccharins. Cultivos Tropicales. 2015;36(Suppl. 1):111-29. [ Links ]
12. Cochran WG, Cox GM. Diseños experimentales. Trillas ^ eD. FDF; 1990. [ Links ]
13. Winarto B, Rachmawati F, Setyawati AS, da Silva JAT. Leaf-derived organogenesis in vitro for mass propagation of lisianthus (Eustoma grandiflorum (Raf.) Shinn. Emirates Journal of Food and Agriculture. 2015;495-501. [ Links ]
14. Pop R, Cantor M, Buta E, Csete I. In vitro plant propagation and crop improvement in Lisianthus Lisianthus russelianus Hook.). Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Horticulture. 2016;73(2):168-74. [ Links ]
15. Sotomayor León EM, Escobar Tolosa MR, Rosas Guerra CA. Propagación del Lisianthus cv. Azul por esquejes en macetas de turba bajo nebulización, con distintas concentraciones de ácido ß-indolbutírico, en el Valle de Azapa. IDESIA (Chile). 2012;29(1):99-102. [ Links ]
16. Posada-Pérez L, Padrón-Montesinos Y, González-Olmedo J, Rodríguez-Sánchez R, Barbón-Rodriguez R, Norman-Montenegro O, et al. Efecto del Pectimorf(r) en el enraizamiento y la aclimatización in vitro de brotes de papaya Carica papaya L.) cultivar Maradol Roja. Cultivos Tropicales. 2016;37(3):50-9. [ Links ]
17. Izquierdo H, Diosdado E, Cepero MCG, de la C Núñez M, Cabrera JC, Hernández RM, et al. Contributions to knowledge of the functioning of national bioestimulators in plant biotechnology processes. Biotecnología Aplicada. 2016;33(3):3511-6. [ Links ]
18. Katz I. Fertirrigação com diferentes doses de nitrogênio, em plantas de Eustoma grandiflorum (Raf.) Shinn.). 2004. [ Links ]
19. Izquierdo Oviedo H, Alcaraz Meléndez L, Rodríguez-Álvarez M. Micropropagación de chiltepín Capsicum annuum L. cv.'Glabriusculum') mediante el empleo de una oligosacarina de origen péctico. Acta Universitaria. 2017;27(5):34-43. [ Links ]
20. García-Águila L, Rodríguez M, Edirisinghage KD, Bernal ILM, La M, Pérez M, et al. Formación de minitubérculos de cuatro variedades cubanas de Solanum tuberosum L. en casa de cultivo. Biotecnología Vegetal. 2016;16(4). [ Links ]
21. Reyes AE, Pupo JJS, García MB, Paneque OSG, Pérez JLP, Rosabal LF. Evaluación de plantas de Curcuma longa L. obtenidas por cultivo de tejidos en condiciones de organopónico. Revista Colombiana de Biotecnología. 2012;14(2):196-202. [ Links ]
22. Ahmad H. Phenotypic screening of lisianthus eustoma grandiflorum lines for production in bangladesh. 2017. [ Links ]
23. Uddin AJ, Roni MZK, Islam MS, Ona AF, Sarker MS, Shimasaki K. Study on growth, flowering and seed production of eight nandini Eustoma grandiflorum varieties. International journal of business, social and scientific research. 2015;3(1):25-9. [ Links ]
24. Uddin A, Taufique T, Ona AF, Shahrin S, Mehraj H. Growth and flowering performance evaluation of thirty two chrysanthemum cultivars. Journal of Bioscience and Agriculture Research. 2015;4(01):40-51. [ Links ]
25. López-Pérez CA, Valdez-Aguilar LA, Robledo-Torres V, Mendoza-Villarreal R, Castillo-Gonzalez AM. El calcio imparte tolerancia a alta conductividad eléctrica en Lisianthus Eustoma grandiflorum Raf. Shinn.). Revista Mexicana de Ciencias Agrícolas. 2014;5(7):1193-204. [ Links ]
26. Izquierdo Oviedo H, González MC, Nuñez Vázquez M. Bioestimuladores del crecimiento para la micropropagación del banano. Académia Española; 2017. [ Links ]
Received: December 11, 2019; Accepted: November 16, 2020











 text in
text in 



