Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Cultivos Tropicales
versão impressa ISSN 0258-5936versão On-line ISSN 1819-4087
cultrop vol.42 no.1 La Habana jan.-mar. 2021 Epub 30-Mar-2021
Original article
Effects of bioactive products on Cicer arietinum L. plants
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
The use of biostimulants and biofertilizers in agriculture has increased in the last years. The objective of this work was to determine the Spirufert biofertilizer influence on chickpea yield and the soil chemical properties and the effect of its joint application with Biobras-16® on the yield. In a first experiment, inoculated seeds with Azofert® before sowing, of BS70 cultivar were used. Three treatments were applied: T1 (Azofert®), T2 (Azofert® + one leaf spray with Spirufert) and T3 (Azofert® + two leaf sprays with Spirufert). In the second, N27 and N38 cultivars were used and two foliar sprays were made with a mixture of Spirufert and Biobras-16®. In the first experiment, it was shown that, when performing two applications (T3) there were differences with the control treatment; in the number of pods, grains and grain mass per plant and the yield. Although it did not differ from the treatment where only one application (T2) was made, there were also no differences in the N, P, K concentrations of the grains between the treatments. A decrease in the magnesium concentration (2.2 cmol kg-1 compared to 0.5 cmol kg-1 of the control treatment) and an increase in the phosphorus concentration (107.5 mg kg-1 compared to 89 mg kg-1 of the control treatment) were found in the soil, in the area where the biofertilizer treatments were. The foliar spray of Spirufert with Biobras-16® increased the number of pods per plant, number and weight of grains per plant and the yield of N27 and N38 cultivars, this latter with increases of 21 and 28 %, respectively.
Key words: brassinosteroids; chickpea; Spirulina; vinasse
INTRODUCTION
Chickpea (Cicer arietinum L.), is a legume of interest that is cultivated in the world, constituting a rich source of proteins, carbohydrates and minerals 1) and in Cuba, in recent years, its production has gained importance 2,3.
At present, at an international level, the use of plant extracts is being resumed to increase agricultural yields 4 and within these extracts, products formulated with algae have been widely used as “plant biostimulators”, achieving good results in increased production 5.
Reports of the cyanobacteria use have shown that these microorganisms contribute to the biological nitrogen fixation, improve the availability of nutrients and help the structure and function of the soil 1. Others have expressed the benefits of using these to improve growth, nutrient consumption and bifortification in crops such as wheat (Triticum aestivum), cotton (Gossypium spp.), Corn (Zea mays), Chinese beans (Phaseolus aureus) 6 and chickpea (Cicer arietinum L.) 7.
Spirulina (Arthrospira platensis) is an algae from the cyanobacteria group, whose use has been widely exploited in biotechnology field 8,9; However, the sustainable management of agricultural production has made it possible to increase its use in agriculture 10.
Another biostimulant that has great interest in agriculture is Biobras-16®, a formulation that has as an active ingredient an analog of brassinosteroids synthesized in Cuba. This has as main effects: increasing yields, increasing crop quality and stimulating plant tolerance plants to environmental stress conditions (water deficit, salinity in the soil and high temperatures), and being widely used in crops such as rice, corn, soy and tomato 11.
In Cuba, investigations related to the response of various crops to Spirulina extract application and other products in which it is present have been initiated; like Spirufert biofertilizer.
The objective of this research was to determine the Spirufert biofertilizer influence on chickpea yield and soil chemical properties, its most suitable application time and the effect of its joint application with Biobras-16® on this yield.
MATERIALS AND METHODS
Two experiments were carried out in the central area of the National Institute of Agricultural Sciences (INCA), located at 23o 00' 05.7” N 82o 08'35.4” W, in San José de las Lajas municipality, Mayabeque province.
The first experiment was carried out between November 2017 and March 2018. Seeds of the BS-70 cultivar were used, which, prior to sowing, were inoculated with Azofert® (biofertilizer based on the Rhizobium leguminosarum strain, at a cellular concentration 2.8 x 1010 UFC), at a rate of 4.35 mL kg-1 of seed. The sowing was carried out in a Nitisol Ferrallic Lixic soil (eutric alcylic rhodic), to which its chemical properties were determined before sowing (Table 1) and after harvesting (Table 2), following the methodology of some authors 12.
Table 1 Main chemical properties that characterize the soil Nitisol Ferrallic Lixic (eutric alcylic rhodic) before starting the first experiment
| Samples | Na | K | Ca | Mg | P | OM | pH |
|---|---|---|---|---|---|---|---|
| cmol kg-1 | mg kg-1 | g kg-1 | |||||
| M1 | 0.07 | 1.03 | 19.8 | 4.0 | 837 | 4.10 | 7.45 |
| M2 | 0.07 | 1.00 | 18 | 2.5 | 385 | 3.31 | 7.60 |
M1 (sample taken in areas where the biofertilizer was applied and was made up of 20 subsamples) and M2 (sample taken in the area where the control was located and was made up of 10 subsamples). All subsamples were taken from 0 to 0.20 m depth
Table 2 Main chemical properties that characterize the soil Nitisol Ferrallic Lixic (eutric alcylic rhodic) at the first experiment end
| Samples | Na | K | Ca | Mg | P | MO | pH |
|---|---|---|---|---|---|---|---|
| cmol kg-1 | mg kg-1 | g kg-1 | |||||
| M1 | 0.075 | 0.84 | 18.5 | 1.8 | 944.5 | 4.30 | 7.2 |
| M2 | 0.07 | 0.80 | 17 | 2.0 | 474.0 | 3.91 | 7.2 |
M1 (sample taken in areas where the biofertilizer was applied and was made up of 20 subsamples) and M2 (sample taken in the area where the control was located and was made up of 10 subsamples). All subsamples were taken from 0 to 20 cm deep
The sowing distance used was 0.75 x 0.20 m. It is important to note that during the entire crop cycle the plants did not receive mineral fertilization or any other product that was not the Spirufert biofertilizer. Weed control was carried out manually and irrigation was applied according to crop needs.
During the crop cycle, the average maximum and minimum temperatures were 28.5 and 17.3 °C, respectively. The accumulated rainfall during this cycle was 192.7 mm, occurring 61.8 % during the vegetative stage and the rest (38.2 %) in the reproductive stage, according to data from Tapaste Meteorological Station, located 500 m from the experimental area.
The biofertilizer called Spirufert was used, which consists of an aqueous suspension of Spirulina jelly (64 %) and vinasse (36 %) and which was supplied by the Spirulina Base Business Unit, Zaragoza, San José de las Lajas, Mayabeque province, belonging to the LABIOFAM SA Company. The treatments were conformed as follows:
T1- Azofert® (Control)
T2- Azofert® + Spirufert foliar spray (3.7 L ha-1) 30 days after sowing
T3- Azofert® + Spirufert foliar spray (2.3 L ha-1) 13 days after sowing + Spirufert foliar spray (3.7 L ha-1) 30 days after sowing.
The sprays were carried out in the early hours of the morning with a backpack of 16 L capacity, checking that the product volume to be applied per plot wet the foliage of the plants well to the point of dripping. In the first spraying of the T3 treatment, it was ensured that the soil was well moistened. Each treatment was applied in two plots composed of five rows of 20 m long each, which is equivalent to an area of 75 m2 (3.75 x 20 m) per plot.
At the experiment end (120 days after sowing), 30 plants were randomly sampled per treatment and the following indicators were evaluated: number of pods per plant, pod percentage with more than one grain, number and mass of grains per plant and 100 grain mass. The agricultural yield was estimated from the production obtained in each plot and was expressed in t ha-1. In addition, N, P, K concentration in the grains was determined, following some authors´ methodology 12.
The second experiment was carried out between the end of November 2018 and February 2019. Seeds of Cuban N27 and N38 cultivars were used, which were not inoculated and were sown in a soil Nitisol Ferralic Lixic (eutric alcylic rhodic) soil.
30 m2 (1.5x20 m) were selected for each of the cultivars under study, to which two foliar sprays (46 and 61 days after sowing) of the mixture of Biobras-16® and Spirufert were made. (1: 2 000 v/v) at a rate of 1.5 L ha-1. The sprays were carried out in a similar way to that described in the first experiment. An area of equal size (30 m2) of each cultivar was used as a control treatment without application.
The plants did not receive mineral fertilization during the crop cycle and weed control, and irrigation was carried out in a similar way to that described in the previous experiment.
During the crop cycle, the average maximum and minimum temperatures were 27.3 °C and 16.5 °C, respectively. The accumulated precipitation during the crop cycle was 108.5 mm, occurring 54.7 % during the vegetative stage and the rest (45.3 %) in the reproductive stage, according to data collected at the same Weather Station.
At the end of the experiment (110 days after sowing), 30 plants per treatment were randomly sampled in each cultivar under study and the same performance evaluations and their components described in the previous experiment were performed.
In both experiments, a random sampling was carried out and the data were processed by calculating the means, the standard deviation and the 95 % confidence interval, with the aim of discriminating differences between the means. For this, the statistical program SPSS was used and the figures were made with the Microsoft Excel 2010 program.
RESULTS AND DISCUSSION
From the yield indicators evaluated, the application of biofertilizer did not modify neither the percentage of pods with more than one grain nor the mass of 100 grains. However, the treatment where two sprays with Spirufert (T3) were performed, was the best, by increasing the rest of the components compared to the control treatment, although this treatment did not differ significantly from the T2 treatment where only one spray was performed (Figure 1).
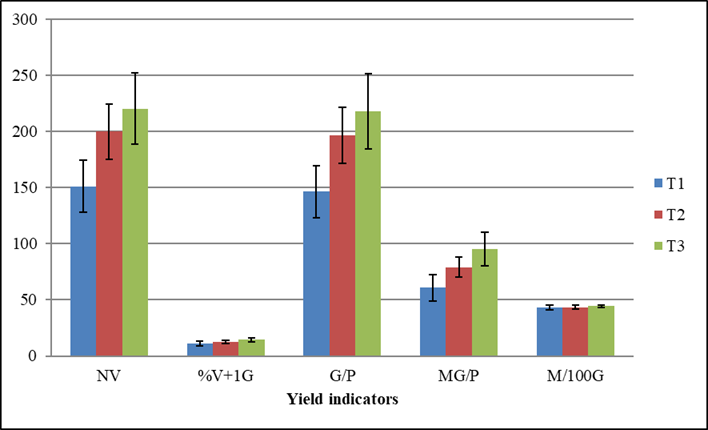
T1 (Control treatment), T2 (Foliar spray with Spirufert at 30 days), T3 (Foliar spray with Spirufert at 13 and 30 days), NV (Number of pods per plant), % V+1G (percent of pods with more than one grain per plant), G/P (Number of grains per plant), MG/P (Mass of grains per plant, g), M100G (Mass of 100 grains, g). The bars represent the confidence interval at α =0.05
Figure 1 Foliar sprays effect with Spirufert biofertilizer (Spirulina + Vinasse) on the yield indicators of chickpea plants cv. BS70
It was also possible to verify that when performing two foliar sprays (T3), plant yield increased, exceeding by 57.7 % the yield obtained by the control treatment plants (T1) and by 20.6 % that obtained by the plants that received a foliar application only (T2), although without significant difference with this last treatment (Figure 2).
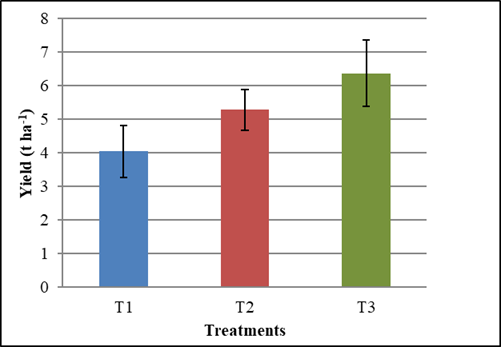
T1 (Control treatment), T2 (Foliar spraying with Spirufert at 30 days), T3 (Foliar spraying with Spirufert at 13 and 30 days). The bars represent the confidence interval at α = 0.05
Figure 2 Biofertilizer Spirufert effect (Spirulina + Vinasse) on chickpea plant yield of cv. BS70
In chickpea crop, the yield varies greatly, depending on the cultivar and the edaphoclimatic conditions of the place where the crop is developed and it can range, for example, from 0.65 t ha-1 for cv. Jamu-96 13, 1.54-1.90 t ha-1 for cv. N-29 13,14 and up to 3,26 t ha-1 for cv. N-27 13.
The results in this work confirm those previously reported by other authors in legumes, who have found that the foliar application of a Spirulina aqueous extract in beans increased the quantity and quality of seeds 15. The extract application to algae base in the carita bean (Vigna unguiculata Lin) increased the number of pods per plant, the mass of 100 grains and the yield 16. An extract application of the brown alga Ecklonia maxima in soybean plants stimulated growth, increased yield, increasing seed number, 1 000 seed mass and improved seed quality by increasing their lipid and protein content 17.
In addition, in other crops, it has been found that rice plant treatment with blue-green algae increased grain production 18) and that when Spirulina extracts were applied to eggplant plants, fruit production increased 19.
The increase in performance induced by the T3 treatment in this work could be positive influence result that Spirufert caused on Azofert® microbial activity and on the photosynthesis process. It has been reported that formula application of a based on Mesorhizobium ciceri and Anabaena family cyanobacteria, it had a positive influence on microbial activity and therefore, on biological nitrogen fixation; in chlorophyll content and available nitrogen in the soil, which resulted in an increase in agricultural yield 1. On the other hand, it has also been reported, for example, in vine cultivation, that the application to the soil and foliage of these algae extracts increased CO2 assimilation rate by plants, which subsequently had an impact on yield 20.
Parallel to this, Spirufert, given its composition, can provide metabolites and nutrients that are essential for plants. It is known that Spirulina has approximately 60-70 % of its dry mass in proteins 21 and essential polyunsaturated fatty acids, vitamins 8, xanthines, phycobiliproteins 8,22, carbohydrates, nitrogen, phosphorus have been identified. Besides, potassium, calcium, iron, manganese, zinc 10, great wealth of α- and ß carotenes 8,23, phycocyanin, considerable amounts of α-linolenic acid, a high phytohormone concentration, trace elements, antioxidants and polysaccharides, chlorophyll a, xanthophylls and lipids 24. In vinasse there are macro and micronutrients such as Ca, Mg, Na, K, Fe, Mn, Zn and Cu 25, moderate amounts of nitrogen, phosphorus 26) and organic matter 25,26.
Despite these results, further research should be done in this regard; to define whether the applications made at two times in T3 treatment (13 and 30 days after sowing) are the most effective to increase the yield in this crop. Also, if it is possible to use another application form such as seed pelletization, direct application to the soil or soil application combination and foliar spraying as has been reported by other authors 4,5,27-29.
Different from the results obtained in some yield components and yield, between the treatments with Spirufert biofertilizer and the control treatment there were no differences in N, P, K concentrations of grains (Table 3).
Table 3 Application effect of Spirufert biofertilizer (Spirulina+Vinasse) on N, P, K concentrations of chickpea plant cv. BS70 grains
| Treatments | N (%) | P (%) | K (%) |
|---|---|---|---|
| T1 | 2.69±0.10 | 0.59±0.03 | 0.68±0.09 |
| T2 | 2.78±0.11 | 0.64±0.06 | 0.71±0.05 |
| T3 | 2.73±0.06 | 0.62±0.04 | 0.72±0.11 |
T1 (Control treatment), T2 (Foliar spray with Spirufert at 30 days), T3 (Foliar spray with Spirufert at 13 and 30 days)
When observing the results shown in the table, it is considered that it should be studied in depth, if application moment and Spirufert concentration could be influencing that there is no response in N, P, K concentrations of grains.
Some authors have found that even though extracts based on algae have a NPK lower content in relation to chemical fertilizers. They stimulate crop growth in a similar way to chemical fertilizer, because they have higher amounts of other elements (calcium, iron, manganese, zinc and selenium) that help to moderate the amounts of nutrients required by plants 10. In addition, in different publications, influence is on diverse mineral concentrations observed in leaves and fruits; however, this response was not in this work observed.
For example, foliar application of a Spirulina aqueous extract in beans, stimulated chlorophyll concentrations, nitrogen, phosphorus and potassium of the leaves 15. On the other hand, when evaluating extract effect based on algae with trade names Agrostemin, Phyllum, Fertimar and Ecoalga, on beans (Phaseolus vulgaris L.) cv Jade, it was observed that K foliar concentration in pods showed statistical differences 30.
However, in a study where Spirulina extracts were applied to eggplant plants, although fruit production increased, N, P, K and Na foliar levels were not affected 19.
Table 4 shows the variations that showed soil chemical properties used in the first experiment once the harvest was finished, in the areas where the biofertilizer treatments were applied and in the area where the control treatment was.
Table 4 Property variations that characterize the Nitisol Ferrallic Lixic (eutric alcylic rhodic soil, FAO 2008) used in the experiment
| Samples | Na | K | Ca | Mg | P | OM | pH |
|---|---|---|---|---|---|---|---|
| cmol kg-1 | mg kg-1 | g kg-1 | |||||
| M1 | +0.005 | -0.19 | -1.3 | -2.2 | +107.5 | +0.2 | -0.25 |
| M2 | 0.00 | -0.2 | -1.0 | -0.5 | +89 | +0.6 | -0.4 |
M1 (sample corresponding to the areas where the biofertilizer was applied) and M2 (sample corresponding to the area where the control treatment was located), (-decrease in concentrations, + increase in concentrations)
The greatest differences in the variation between the areas where biofertilizer was applied and the area where the control treatment was, were observed in Mg and P concentrations, where there was a decrease and a greater increase in magnesium and phosphorus levels, respectively in the soil area where the biofertilizer was applied.
There are reports referring to the influence of cyanobacteria on the availability of nutrients in the soil 1.
The decrease in magnesium levels may be because with the biofertilizer application, physiological process activation in plants where magnesium is required increases. For example, the possible increase in chlorophyll concentration in leaves, which Mg demand as it is part of the molecule and this, of course, may be related to greater photosynthetic activity, effects that have been described by other authors when applying algae extracts 1,20.
The greatest increase in phosphorus concentrations in the area where biofertilizer was applied may be due to phosphorus contribution from the biofertilizer and minerals that help to moderate amounts of nutrients required by the plants.
Once Spirufert biofertilizer efficacy had been demonstrated in chickpea crop, to carry out an experiment was decided. It was used but in combination with Biobras-16® at a slightly lower dose (3 L ha-1) but divided into two moments of application (46 and 61 days after sowing, that is, in the moments of pre-flowering and flowering of the crop.
Although in the first experiment, the best results were obtained when spraying was carried out at 13 days and 30 days after sowing. In this experiment it was decided to use two other cultivars (N-27 and N-38) of chickpea. Two foliar sprays were also carried out, but at other crop cycle times, taking into account that in the case of Biobras-16® the best results have been obtained when applications are made prior to and during the reproductive phase 11.
When analyzing the results, it can be observed that the combined Spirufert and Biobras-16® application significantly increased grain production of the two cultivars under study, which had an impact on grain mass per plant (Figure 3) and therefore, in estimated crop yield (Figure 4).
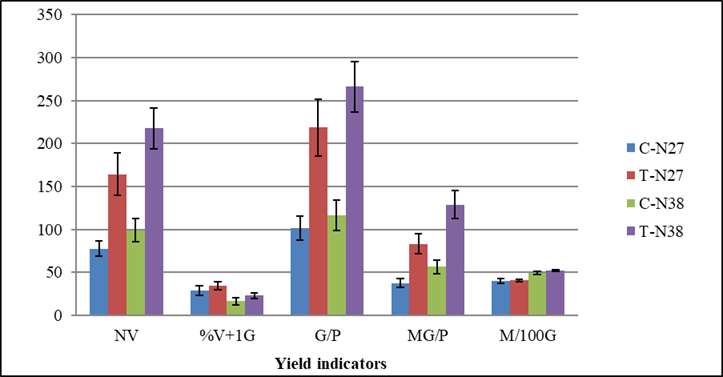
C-N27 (Treatment control cultivar N27), T-N27 (treatment with the mixture cultivar N27), C-N38 (Treatment control cultivar N38). T-N38 (treatment with the mixture cultivar N38), NV (Number of pods per plant), % V + 1G (percent of pods with more than one grain per plant), G / P (Number of grains per plant), MG/P (Mass of grains per plant, g), M100G (Mass of 100 grains, g). The bars represent the confidence interval at α = 0.05
Figure 3 Influence of two foliar sprays with a Spirufert (Spirulina + Vinasse) and Biobras-16® mixture on yield indicators of chickpea plants, N27 and N38 cultivars
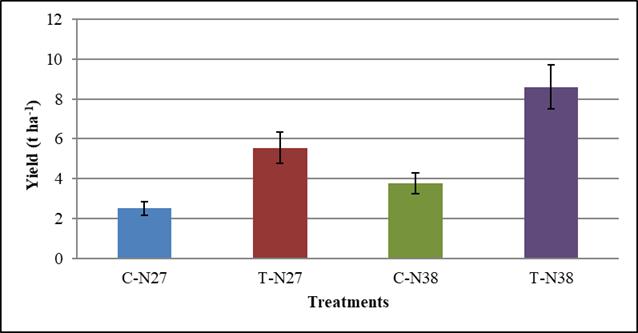
C-N27 (Treatment control cultivar N27), T-N27 (treatment with the mixture cultivar N27), C-N38 (Treatment control cultivar N38), T-N38 (treatment with the mixture cultivar N38). The bars represent the confidence interval at α = 0.05
Figure 4 Influence of two foliar sprays with a Spirufert (Spirulina + Vinasse) and Biobras-16® mixture on the estimated yield of chickpea plants, N-27 and N-38 cultivars
The increases in yield reached values of 21 and 28 %, for N27 and N38 cultivars, respectively. This demonstrates the two foliar spray effectiveness made with the Spirufert and Biobras-16® mixture.
It is known that Biobras-16® has as an active ingredient a spirostanic analog of brassinosteroids, which has been shown to act as a growth regulator in plants, which is why it is characterized by stimulating various physiological processes 11. In this way, studies carried out on beans have shown that foliar spraying with Biobras-16® increased the length and stem diameter, as well as the number of pods and grains per plant and therefore, the commercial yield 31. Similar results were obtained with the foliar spray of Biobras-6 (formulation that has as active ingredient another spirostanic analog of brassinosteroids) in three soybean varieties inoculated with Bradyrhizobium japonicum and Glomus clarum32. This response may be related to root growth stimulation and nodule number induced by foliar spraying, in the flowering phase, of natural brassinosteroids such as homobrasinolide and 24-epibrasinolide, reported by other authors 33.
On the other hand, spraying bean seeds with Biobras-16®, prior to inoculation with Azofert®, significantly increased crop yield 34, which denoted this product effectiveness as a yield biostimulator in this crop.
From the above, it can be inferred that the effect found was expected, although it remains to be determined whether the combined application of Biobras-16® and Spirufert increased or not the effect of Biobras-16® alone and whether this effect was additive or synergistic. It is of great importance today taking into account that both products are national and it is necessary to increase the production of this crop in the country with the use of sustainable production technologies that substitute imports.
CONCLUSIONS
Foliar spraying with Spirufert biofertilizer at two moments of the vegetative phase increased the chickpea crop yield; although there was no influence on N, P, K levels in the grains. In addition, the treatment modified P and Mg levels in the soil.
Foliar spraying with the Spirufert and Biobras-16® mixture produced a significant increase in yield and its components in two chickpea cultivars; which suggests that it is possible to achieve an additive or synergistic effect on plant performance with the mixture of these two national products.
ACKNOWLEDGMENT
To the Spirulina Base Business Unit, Zaragoza, San José de las Lajas, Mayabeque province, belonging to the LABIOFAM S.A Company, for supplying the Spirufert biofertilizer and thus contributing to research in order to achieve greater results in our agriculture.
REFERENCES
1. Bidyarani N, Prasanna R, Babu S, Hossain F, Saxena AK. Enhancement of plant growth and yields in Chickpea (Cicer arietinum L.) through novel cyanobacterial and biofilmed inoculants. Microbiological Research. 2016;188-189:97-105. doi:10.1016/j.micres.2016.04.005 [ Links ]
2. Doimeadiós Reyes Y, Sánchez Llanes A. Productividad y eficiencia en la economía cubana: una aproximación empírica. Economía y Desarrollo. 2015;153:90-107. [ Links ]
3. Cárdenas-Travieso RM, Lamz-Piedra A, Ortiz-Pérez R. Comportamiento morfoagronómico de genotipos promisorios de garbanzo (Cicer arietinum L.). Cultivos Tropicales. 2018;39(2):89-95. [ Links ]
4. Povero G, Mejia JF, Di Tommaso D, Piaggesi A, Warrior P. A systematic approach to discover and characterize natural plant biostimulants. Frontiers in plant science. 2016;7:435. [ Links ]
5. Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants in horticulture. Scientia Horticulturae. 2015;196:39-48. [ Links ]
6. Anitha L, Bramari GS, Kalpana P. Effect of supplementation of Spirulina platensis to enhance the zinc status in plants of Amaranthus gangeticus, Phaseolus aureus and tomato. Advances in Bioscience and Biotechnology. 2016;7(6):289-99. [ Links ]
7. Beghdady MS, Selim D, Nassar R, Salama A. Influence of foliar spray with seaweed extract on growth, yield and its quality, profile of protein pattern and anatomical structure of chickpea plant (Cicer arietinum L.). Middle East Journal of Applied Sciences. 2016;6(1):207-21. [ Links ]
8. Papadaki S, Kyriakopoulou K, Tzovenis I, Krokida M. Environmental impact of phycocyanin recovery from Spirulina platensis cyanobacterium. Innovative Food Science & Emerging Technologies. 2017;44:217-23. [ Links ]
9. Spolaore P, Joannis-Cassan C, Duran E, Isambert A. Commercial applications of microalgae. Journal of bioscience and bioengineering. 2006;101(2):87-96. [ Links ]
10. Wuang SC, Khin MC, Chua PQD, Luo YD. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal research. 2016;15:59-64. [ Links ]
11. Núñez Vázquez M, Reyes Guerrero Y, Rosabal Ayán L, Martínez González L. Análogos espirostánicos de brasinoesteroides y sus potencialidades de uso en la agricultura. Cultivos Tropicales. 2014;35(2):34-42. [ Links ]
12. Paneque-Pérez VM, Calaña J, Calderón M, Borges Y, Hernández T, Caruncho M. Manual de técnicas analíticas para análisis de suelo, foliar, abonos orgánicos y fertilizantes químicos. Ediciones INCA; 2010. [ Links ]
13. Álvarez Rodríguez R. Comportamiento agroproductivo de cuatro cultivares de garbanzo (Cicer arietinum, L.) en condiciones edafoclimáticas de la zona sur del municipio Jesús Menéndez, Las Tunas. [Las Tunas, Cuba]: Vladimir Ilich Lenin; 2013. 35 p. [ Links ]
14. Delgado MA, Pino R, Izquierdo VE. Evaluación del comportamiento del garbanzo (C. arietinum) variedad nacional l-29 en condiciones de suelo arenoso. Avances. 2000;2(2). [ Links ]
15. Seif YIA, El-Miniawy SE-DM, El-Azm NAA, Hegazi AZ. Response of snap bean growth and seed yield to seed size, plant density and foliar application with algae extract. Annals of Agricultural Sciences. 2016;61(2):187-99. [ Links ]
16. Escobar-Oña WS, Tafur-Recalde VL, Pazmiño-Mayorga JA, Vivas-Vivas RJ. Respuesta del cultivo de fréjol caraota (Phaseolus vulgaris L.) a la aplicación foliar complementaria de tres bioestimulantes. Dominio de las Ciencias. 2017;3(3):556-71. [ Links ]
17. Kocira S, Szparaga A, Kubon M, Czerwinska E, Piskier T. Morphological and biochemical responses of Glycine max (L.) Merr. to the use of seaweed extract. Agronomy. 2019;9(2):93. [ Links ]
18. Paliwal C, Mitra M, Bhayani K, Bharadwaj SV, Ghosh T, Dubey S, et al. Abiotic stresses as tools for metabolites in microalgae. Bioresource technology. 2017;244:1216-26. [ Links ]
19. Dias GA, Rocha RHC, Araújo JL, Lima JF, Guedes WA. Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semina: Ciências Agrárias. 2016;37(6):3893-902. [ Links ]
20. Zermeño-González A, Mendez-López G, Rodríguez-García R, Cadena-Zapata M, Cárdenas-Palomo JO, Catalán-Valencia EA. Biofertilización de vid en relación con fotosíntesis, rendimiento y calidad de frutos. Agrociencia. 2015;49(8):875-87. [ Links ]
21. Soni RA, Sudhakar K, Rana RS. Spirulina from growth to nutritional product: A review. Trends in food science & technology. 2017;69:157-71. [ Links ]
22. Campanella L, Crescentini G, Avino P. Chemical composition and nutritional evaluation of some natural and commercial food products based on Spirulina. Analusis. 1999;27(6):533-40. [ Links ]
23. Pagnussatt FA, de Lima VR, Dora CL, Costa JAV, Putaux J-L, Badiale-Furlong E. Assessment of the encapsulation effect of phenolic compounds from Spirulina sp. LEB-18 on their antifusarium activities. Food Chemistry. 2016;211:616-23. [ Links ]
24. Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, et al. Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation. 2009;28(4):386-99. [ Links ]
25. Leal I, Chirinos E, Leal M, Morán H, Barrera W. Caracterización fisicoquímica de la vinaza del Agave cocui y su posible uso agroindustrial. Multiciencias. 2003;3(2). [ Links ]
26. Salamanca Romero CA. Efecto de las fuentes orgánicas obtenidas de los subproductos agroindustriales de la caña de azúcar (Saccharum officinarum L) y el plátano (Musa spp.) sobre la actividad microbiana y enzimático en el suelo [Maestría]. [Colombia]: Universidad Nacional de Colombia; 2008. 63 p. [ Links ]
27. Renuka N, Guldhe A, Prasanna R, Singh P, Bux F. Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnology advances. 2018;36(4):1255-73. [ Links ]
28. López BC. Enzimas-algas: posibilidades de su uso para estimular la producción agrícola y mejorar los suelos. Terra Latinoamericana. 1999;17(3):271-6. [ Links ]
29. Grzesik M, Romanowska-Duda Z, Kalaji HM. Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L.) plants under limited synthetic fertilizers application. Photosynthetica. 2017;55(3):510-21. [ Links ]
30. Gutiérrez Gavonel YK. Extractos de algas marinas en el rendimiento y calidad de vainita (Phaseolus vulgaris L.) bajo condiciones de La Molina [Grado]. [Lima-Perú]: Universidad Nacional Agraria ¨La Molina¨; 2016. [ Links ]
31. Vuelta-Lorenzo DR, Vidal-Cuevas Y, Rizo-Mustelier M, Bell-Mesa T, Molina-Lores LB. Efecto del brasinoesteroide foliar (Biobras 16) sobre el crecimiento y la producción del cultivo de frijol (Phaseolus vulgaris l.). Ciencia en su PC. 2017;(3):1-12. [ Links ]
32. Corbera J, Núñez M. Evaluación agronómica del análogo de brasinoesteroides BB-6 en soya, inoculada con Bradyrhizobium japonicum y HMA, cultivada en invierno sobre un suelo Ferralsol. Cultivos Tropicales. 2004;25(3):9-13. [ Links ]
33. Upreti KK, Murti GSR. Effects of brassmosteroids on growth, nodulation, phytohormone content and nitrogenase activity in French bean under water stress. Biologia Plantarum. 2004;48(3):407-11. [ Links ]
34. Martínez-González L, Reyes-Guerrero Y, Pérez-Domínguez G, García MCN, Núñez-Vázquez M de la C. Influencia del Biobras-16(r) y el QuitoMax(r) en aspectos de la biología de plantas de frijol. Cultivos Tropicales. 2018;39(1):108-12. [ Links ]
Received: December 04, 2019; Accepted: November 04, 2020











 texto em
texto em 



