My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Cultivos Tropicales
Print version ISSN 0258-5936On-line version ISSN 1819-4087
cultrop vol.42 no.3 La Habana July.-Sept. 2021 Epub Sep 30, 2021
Original article
Induction of signals in Rhizobium leguminosarum bv. viciae and its biological activity in pea (Pisum sativum L.)
1Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
2Fyteko, Allée de la Recherche 4, 1070 Anderlecht, Belgium
3Unité Biotechnologie-Materia Nova A.S.B.L., Rue des Foudriers 1, 7822 Ghislenghien, Belgium
4Institute Biosciences, University of Mons, Belgium
5Empresa Rizobacter S.A., Ruta Nº 32, Km. 1,5 Parque Industrial de Pergamino, Buenos Aires, Argentina
6Empresa Indrasa Biotecnología S.A., Av. Ltdor. Gral. San Matín 2241, Río Cuarto, Córdoba, Argentina
Pea is a versatile plant, used in different ways and for different purposes, due to its high nutritional value. Its symbiotic interaction with bacteria of the rhizobia family guarantees significant amounts of nitrogen, from biological fixation, which allows it to grow in environments lacking this element. The agronomic and environmental importance of this symbiosis has required technology improvement for the production and inoculant application, so this work set out to evaluate the induction effect of a Rhizobium leguminosarum bv. viciae strain in the generation of signals in the inoculant and its biological activity on pea plants. Daidzein was used as an inducer at a final concentration of 5 µM. The lipidic fraction in the inocula was extracted with n-butanol and analyzed by high performance liquid chromatography and gas chromatography coupled to a mass spectrometer. Biological activity was evaluated in one experiment under controlled conditions, two field experiments, and one extensive trial. The results showed that in the inoculant induced with the flavonoid, a higher quantity and diversity of molecules related to the symbiotic interaction was detected. Similarly, the induced inocula showed a higher positive effect on nodulation (14.8 vs 8.7) and the yield of pea plants, with 468 and 125 kg ha-1 of seeds above the inoculated treatment without inducing in the experiments in plots and 746 kg ha-1 in the extensive trial.
Key words: biological fixation; nodulation; communication; legumes
INTRODUCTION
Pea (Pisum sativum L.) is a grain legume, characterized by its high nutritional value and its use in human and animal nutrition. Its seeds contain between 18 and 20 % of dry matter, of which 10 to 12 % are carbohydrates and between 5 and 8 % are proteins 1. Pea beans can be used for immediate consumption or canned for long-term storage 2. This crop can also be used as green fodder, hay, silage or flour and as green manure 3.
As a legume, the pea has another important characteristic, related to the ability to fix atmospheric nitrogen in symbiosis with bacteria of the rhizobia family 4, specifically with the species R. leguminosarum bv. viciae5. This symbiosis provides significant amounts of N to the soil for successor crops and it is suggested that inoculation with these bacteria can produce similar yields to the application of 200 kg of N ha-1 (2.
The application of large amounts of inorganic fertilizers in intensive production systems 6,7) represents a polluting burden for the environment and high costs that many countries cannot afford. Achieving similar or higher yields with low inputs and organic products represents a challenge for sustainable agriculture. Symbiotic fixation of N is a promising alternative in this regard 8,9.
The inoculation technologies of various beneficial microorganisms in seeds constitute an economical agronomic practice, recommended in agriculture. Successful inoculation will depend on several factors, including the ability of the introduced strain to compete with the existing native population and achieve greater infection, nodulation, and N contribution 10.
The symbiosis established between legume plants and rhizobia responds to an intense exchange of signals from the beginning of the interaction, which allows communication between both symbionts: the macro and the microorganism 11. Seeds release various compounds, including flavonoids, which cause the expression of nod genes in bacteria, which induces the synthesis of Nod factors, essential molecules in the interaction success 12. These compounds have also been related to the chemo-attraction exerted by legumes on rhizobia and with the expression of other genes in these bacteria that regulate the synthesis of exopolysaccharides and the type III secretion system, related to the defensive responses in the host and protein export in nodulation, respectively 13.
High molecular weight fatty acids are structural components of lipoquitooligosaccharides associated with nodulation. Nod factors consist of a backbone of three to five molecules of N-acetyl-glucosamine, which at the amino group of the non-reduced end is acylated with a fatty acid 16-20 C atoms long (C16-C20) 14.
The use of inoculants induced in the synthesis of these signals that govern communication, has made it possible to obtain not only better nodulation and plant performance 15, but also tolerance to stress conditions in the environment 16. For this reason, the present work aimed to evaluate the induction effect of a Rhizobium leguminosarum bv. viciae strain in the generation of signals in the inoculant and its biological activity in pea plants.
MATERIALS AND METHODS
Bacterial culture. Induction with daidzein
A strain of Rhizobium leguminosarum bv viciae was used, known for its symbiosis with the pea. A preinoculum was produced in 50 mL of mannitol yeast extract medium 17 at pH 6.8; to inoculate 600 mL of the same medium without and with daidzein (Sigma), which was added to a final concentration of 5 µM. The inocula were obtained after keeping the flasks at 150 rpm for 48 h, in an orbital shaker at 28 ± 2 ºC of temperature. For each case, the procedure was repeated three times and included three samples from each treatment (uninduced and daidzein-induced).
Extraction of the lipidic fraction
Taking into account that most of signals recognized by their biological activity in the rhizobia-legume interaction are lipids, a selective extraction of these molecules was carried out from the inoculum treated with daidzein (Induced) or not (Control), using 180 mL of n-butanol in each sample. They were placed on an orbital shaker at 150 rpm for 15 min and kept overnight under dark conditions at a room temperature of 25 ± 2 °C. Then the organic phase in each sample was extracted, centrifuged at 12000 g, 10 °C, for 10 minutes. All samples were concentrated by rotary evaporation at 50-80 °C until 2 mL of each were obtained, which were used for the detection of signals produced by the bacteria.
Signal detection. Structural characterization of main components in the lipidic fraction
High performance liquid chromatography analysis, reversed phase
To evaluate the presence of nodulation factors among the metabolites, 10 µL of all lipidic extracts were analyzed by HPLC, using a Waters Symmetry C-18 reversed phase column (46 x 250 mm) of 5 µ particle size, installed on a Waters Alliance HPLC System. The flow rate was 1 mL min-1 and water (A) and acetonitrile (B) were used as solvents, with a gradient: 0-10 min 18 % B, 10-30 min 60 % B, 30-35 min 95 % B, 35-45 min 18 % B. A UV-Waters spectrophotometric detector was used at a wavelength of 214 nm.
Gas chromatography coupled to a mass spectrometer
The volatile fatty acid derivatives were prepared by silylation, using BSTFA (N, O-Bis trimethylsilyl trifluoroacetamide) as a reagent in combination with trimethylchlorosilane (TMCS) (BSTFA+TMCS Kit, Supelco). For the analysis by GC-MS, a gas chromatograph coupled to a Shimadzu GC-MS QP-2010 mass spectrometer was used; system equipped with AOC-20i autoinjector, AOC-20s automatic injector and a direct insertion system controlled by the "GC-MS Solution" software. An Optima 5 MS column (30 µm × 0.25 mm ID, 0.25 µm film thickness) was used. The conditions of the chromatographic analysis were: injector temperature 310 °C, oven temperature 100 °C for six minutes. Subsequently, it was increased to 320 °C at a rate of 20 °C min-1 and held for 5 minutes. The injection volume was 1 µL and the column flow was 0.75 mL min-1, using helium as a stripping gas.
Effect on the pea plant nodulation, under controlled conditions
From the biopreparations obtained, 2 mL were inoculated for every 500 g of Facon variety seeds, which were sown in a sterile soil/vermiculite mixture (1:1), without the application of other nutrients. The plants were grown in a growth chamber, with a photoperiod of 16/8 hours of light/dark, at 28 °C and with 60 % relative humidity, for 35 days. Irrigation was carried out by capillarity with deionized water, maintaining the water regime at approximately 90 % of the field capacity. Nine plants were used per treatment to evaluate the number and dry mass of nodules (mg) per plant.
Effect on plant performance, under field conditions
This study was carried out in experimental fields of the localities Ferré (34º09´S, 61º09´W, altitude 93 m, typical black Mollisol soil, which corresponds to a Feozem soil 18) and Pergamino (33° 53'S, 60 ° 34'W, altitude 56 m, typical Argiudol soil, loamy-silty (Argic Feozem), Buenos Aires province, in Argentina, during the months of July-October 2015. Two of the trials were carried out in plots (in both localities) and an extensive one in Ferré.
The data from the soil analysis in Pergamino were: pH (1: 2.5) = 5.6; N-NO3 (ppm) = 4; P Bray I (ppm) = 19.2; Org. M. (%) = 2.2; Total N (%) = 0.111; Carbon (%) = 1.3. Temperatures (°C) between July-October: 16.4-20.1-20.4-25.0 and rainfall (mm): 33.0-8.0-60.0-111.0.
For Ferré, the data from the soil analysis were: pH (1: 2.5) = 5.6; N-NO3 (ppm) = 13.7; P Bray I (ppm) = 23.6; Org. M. (%) = 2.7; Total N (%) = 0.134; Carbon (%) = 1.6. Temperatures between July-October: 16.3-21.0-20.7-24.8 °C and rainfall: 3.0-4.0-68.0-105.0 mm, respectively.
Seeds of Facon variety were inoculated at a dose of 4 mL per 1000 g of seeds, with a sowing density of 25 seeds m-1 and a direct sowing system (without tillage) in Ferré and conventional sowing (previous tillage of the soil ) in Pergamino. As a predecessor crop, in Ferré soybeans were cultivated, while in Pergamino it was pastures. In all the experiments, the following was used as fertilization: 80 kg ha-1 of Mixture (8-38-0-8 S). At the time of harvest, the yield (kg ha-1) was determined.
Experimental design and statistical processing
The growth chamber experiment was repeated three times. A completely randomized design was used and the normality and homogeneity of the variance was checked in the data, which were analyzed according to Tukey p <0.05.
In the plot experiments a bifactorial analysis was used, taking into account the inoculant factors and localities, with three and two levels, respectively. To discriminate the differences between the means, results were compared using Duncan's Multiple Range Test p <0.05. The Statgraphics Plus program, version 5.1, was used.
RESULTS AND DISCUSSION
Signal detection. Structural characterization of main components in the lipid fraction
The extract composition analysis in n-butanol, corresponding to three independent biological replicates for each treatment, carried out by HPLC and GC-MS is presented in Figures 1 and 2, respectively.
Differences are observed between the induced and non-induced treatments in the peaks obtained and the area (concentration), which was higher when the biopreparation was induced with daidzein (Figure 1 A and B), whose total area was 372 567 175, with respect to non-induced treatment, which was 5 322 255.

Data represents the average of three replications
Figure 1 Chromatograms obtained by HPLC, retention time (rt) and area of the separated peaks, when analyzing the samples of the non-induced inoculant (A) and the inoculant induced with daidzein (B)
When the samples were analyzed by gas chromatography coupled to a mass spectrometer (Figure 2), several peaks were identified, corresponding to high molecular weight fatty acids such as ecosenoic and stearic acid and other molecules synthesized by the microorganism.
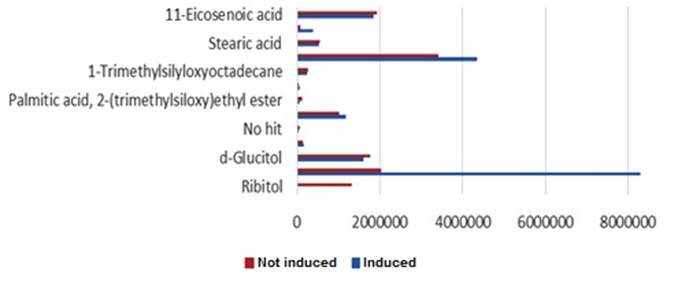
Data represents the average of three replications
Figure 2 Area of peaks corresponding to the chromatograms obtained by GC-MS in the inoculant not induced and induced with daidzein and representing fatty acids and other molecules
The results show an increase in the area of these peaks with the induced treatment. This means that daidzein promotes not only the production of nodulation factors in bacteria, but also other components of a lipid nature, such as fatty acids and other compounds.
In previous works, other authors also found an increase in these high molecular weight fatty acids in the Nod factors produced by symbiotic strains of beans and chickpeas, in the presence of nod gene inducers 15,19. The biological role of nodulation factors is well documented. However, a possible function of the rest of the compounds detected in the nodulation process is unknown. In fact, high molecular weight fatty acids have been reported to exhibit antimicrobial activity and play a role in Rhizobium growth and stress adaptation 20. These fatty acid structures are also components of plant cell membranes and have been shown to accumulate in roots of soybean plants colonized by Bradyrhizobium japonicum21. Some high molecular weight fatty acids are precursors of the synthesis of jasmonic acid, which is essential in the plant's responses to biotic and abiotic stress 22.
Through the methods used to characterize lipid compounds and detect the presence of Nod factors in inoculant samples, these structures were identified to a greater extent in the daidzein-induced inoculant.
Effect on nodulation under controlled conditions
The non-inoculated plants did not form nodules, but regarding the effect of the induced and non-induced treatments on nodulation, a superior effect of the treatment inoculated with the induced inoculant was observed on the number of nodules formed in the main and total roots and also in its mass (Table 1).
Table 1 Number of nodules and nodule dry mass (mg) in non-inoculated control plants (Control) and plants inoculated with the induced or non-induced biopreparation, 35 days after sowing
| Treatment | Nodules number Principal Root pl-1 | Nodules dry mass Principal Root pl-1 | Number of nodules Total pl-1 | Total nodule dry mass Total pl-1 |
|---|---|---|---|---|
| Induced | 3.56 a | 7.67 a | 14.89 a | 19.56 a |
| Not Induced | 0.36 b | 0.73 b | 8.79 b | 13.21 b |
| Control | 0.0 b | 0.0 b | 0.0 c | 0.0 c |
| SE x | 0.35 | 0.89 | 1.24 | 1.02 |
The data represents the average of three repetitions
Similar letters indicate that there are no differences between the treatments, according to Tukey p <0.05; n = 9
These results indicate that the presence of a greater quantity and diversity of signals in the induced inoculant (Figures 1 and 2), stimulated the formation of nodules in the plants. It has been suggested that the perception of signals, such as Nod factors, activate the biosynthetic pathways required for nodulation 23 and that a greater number of these structures means greater biological fixation, as well as a greater nodular mass could indicate more nodular tissue, more bacteroides and more fixation; therefore, greater supply of N to the plant 24.
Effect of inoculants on pea yield, under field conditions
The analysis in plots showed interaction between the factors: inoculants (control, not induced and induced) and localities (Ferré and Pergamino). Higher yields were obtained in the biofertilized treatments (Figure 3), which demonstrates the role of these bacteria in the nitrogen nutrition of the culture. Similar results were obtained by other authors 25.
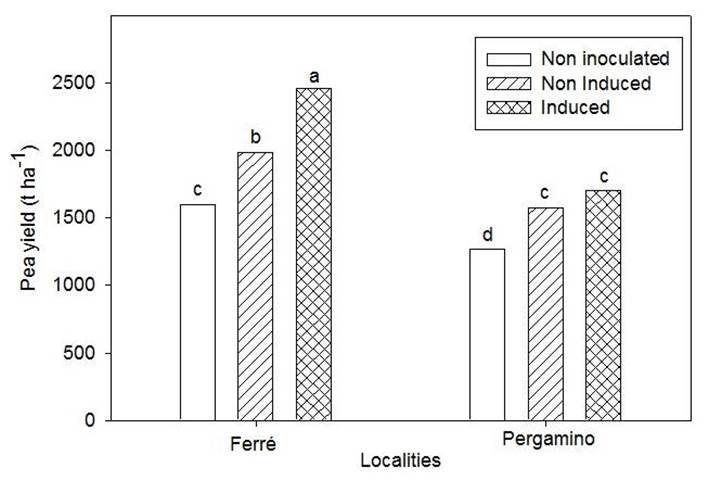
Means with similar letters indicate non-significant differences Duncan p <0.05. ES X = 54.67
Figure 3 Effect of non-inoculated, induced and non-induced treatments on pea yield in two locations
Yields were higher in Ferré, with around 1000 t ha-1 more, compared to Pergamino. Taking into account that the same variety, treatments and cultural attentions were used in both experiments and that the characteristics of the soil and the climate were not significantly different, the difference in yield obtained for both locations could be related to the previous crop and to the type of seeding used. The positive effect of legumes on crop rotation is known 26,27, due to residual rhizobia populations with positive attributes in promoting plant growth and their ability to fix atmospheric nitrogen 28, by facilitating the circulation of soil nutrients and water retention 29 and by reducing nutrient runoff and greenhouse gas emissions 30.
Regarding advantages of direct sowing over conventional soil preparation methods, there are increases in the number of established seedlings 31, water savings 32 and increases in crop yields and quality 33.
When inoculated treatments were compared, a superior yield of the legume was observed in both places when the induced inoculant was used, with 468 and 125 kg ha-1 of seeds, above the inoculated treatment without inducing, for Ferré and Pergamino, respectively. However, significant differences between these inoculated treatments were only observed in Ferré.
In the extensive trial, similar results were found to the experiments in plots, with higher yields in the treatment inoculated with the induced bacteria (2637 kg ha-1), followed by the non-induced treatment, but inoculated (1891 kg ha-1). The control showed the lowest yield value (1501 kg ha-1) (Figure 4).
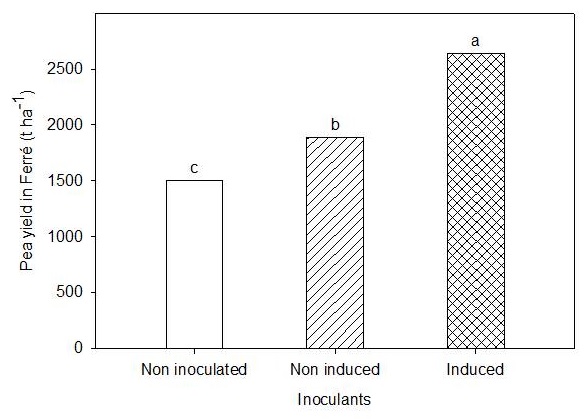
Means with similar letters indicate non-significant differences, according to Duncan p <0.05. ES X = 22.16
Figure 4 Effect of non-inoculated (control), induced and non-induced treatments on pea yield, under extensive field conditions in Ferré
The positive effect found in the nodulation variables (Table 1) translated into higher crop yields under different conditions. Similar results were obtained by other authors for the Bradyrhizobium elkani-soybean, Rhizobium leguminosarum-bean and Mesorhizobium ciceri-chickpea interaction, when the inocula were also induced. The analysis of the chromatographic profiles obtained in these investigations also showed positive differences in the number of peaks and their area; results that corresponded with the number of nodules and later with the yield of these cultures 15,19,34.
The importance of Nod factors in the interaction with legumes is not only related to nodulation and the efficiency in the biological fixation of N under normal conditions and abiotic stress. The similarity of its structure with the chitooligosaccharides derived from the cell wall of fungi and defense activators 35, as well as direct evidence of its role in reducing diseases 36) and other indirect evidence in the activation of defense enzymes 37), represent a participation of these molecules in certain immune responses to invasion by pathogens 38.
CONCLUSIONS
The induction of nodulation genes in Rhizobium leguminosarum bv. viciae, as part of the improvement in the inoculants and their dialogue with the plants, causes the synthesis and excretion of different compounds by the bacteria, which has an impact on greater nodulation and yield in the pea crop.
The development of inoculants and more complex formulations such as those discussed here, allow to improve the symbiosis and guarantee higher levels of N fixation.
BIBLIOGRAFÍA
1. Maiza B, Siles M, Ríos R, Gabriel J. Comportamiento de catorce líneas mejoradas de arveja (Pisum sativum L.) en la zona de Challapata, Oruro. Journal of the Selva Andina Research Society. 2015;6(1):10-22. [ Links ]
2. Muniz AW, COSTA M, de Sá ELS, Fiuza AB, Brose E. Symbiotic efficiency of pea (Pisum sativum) rhizobia association under field conditions. Embrapa Amazônia Ocidental-Artigo em periódico indexado (ALICE). 2017; [ Links ]
3. Jaiswal NK, Gupta AK, Dewangan H, Lavanya GR. Genetic variability analysis in field pea (Pisum sativum L.). Int. J. Sci. Res. 2015;4(1):2006-7. [ Links ]
4. Beiranvand JP, Pourbabaee AA, Shirmardi SP, Alikhani HA, Abbasi AR, Motesharezadeh B. Symbiotic nitrogen fixation, phosphorus and potassium uptake capacity of a number of soybean mutant lines in a calcareous soil. Journal of Agricultural Science & Technology (1008-0864). 2018;20(7):1555-64. [ Links ]
5. Leppyanen IV, Kirienko AN, Dolgikh EA. Agrobacterium rhizogenes mediated transformation of Pisum sativum L. roots as a tool for studying the mycorrhizal and root nodule symbioses. PeerJ. 2019;7:6552. [ Links ]
6. Malhi SS, Johnson EN, Hall LM, May WE, Phelps S, Nybo B. Effect of nitrogen fertilizer application on seed yield, N uptake, and seed quality of Camelina sativa. Canadian Journal of Soil Science. 2014;94(1):35-47. [ Links ]
7. Wile A, Burton DL, Sharifi M, Lynch D, Main M, Papadopoulos YA. Effect of nitrogen fertilizer application rate on yield, methane and nitrous oxide emissions from switchgrass (Panicum virgatum L.) and reed canarygrass (Phalaris arundinacea L.). Canadian Journal of Soil Science. 2014;94(2):129-37. [ Links ]
8. Granda Mora KI. Inoculante a base de una cepa nativa de Rhizobium leguminosarum bv. viciae col6 para la producción de Phaseolus Vulgaris L. en loja, Ecuador [Doctorado]. [Las Villas, Cuba]: Universidad Central´´ Marta Abreu´´; 2017. 143 p. [ Links ]
9. Geurts R, Lillo A, Bisseling T. Exploiting an ancient signalling machinery to enjoy a nitrogen fixing symbiosis. Current opinion in plant biology. 2012;15(4):438-43. [ Links ]
10. Lodeiro AR. Interrogantes en la tecnología de la inoculación de semillas de soja con Bradyrhizobium spp. Revista argentina de microbiología. 2015;47(3):261-73. [ Links ]
11. D. Oldroyd GE. Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nature Reviews Microbiology. 2013;11(4):252-63. [ Links ]
12. Liu C-W, Murray JD. The role of flavonoids in nodulation host-range specificity: an update. Plants. 2016;5(3):33. [ Links ]
13. Gourion B, Berrabah F, Ratet P, Stacey G. Rhizobium-legume symbioses: the crucial role of plant immunity. Trends in plant science. 2015;20(3):186-94. [ Links ]
14. Poinsot V, Crook MB, Erdn S, Maillet F, Bascaules A, Ané J-M. New insights into Nod factor biosynthesis: Analyses of chitooligomers and lipo-chitooligomers of Rhizobium sp. IRBG74 mutants. Carbohydrate research. 2016;434:83-93. [ Links ]
15. Nápoles García MC, Cabrera Pino JC, Onderwater R, Wattiez R, Hernández Forte I, Martínez González L, et al. Señales producidas por Rhizobium leguminosarum en la interacción con frijol común (Phaseolus vulgaris L.). Cultivos Tropicales. 2016;37(2):37-44. [ Links ]
16. Duzan HM, Zhou X, Souleimanov A, Smith DL. Perception of Bradyrhizobium japonicum Nod factor by soybean [Glycine max (L.) Merr.] root hairs under abiotic stress conditions. Journal of Experimental Botany. 2004;55(408):2641-6. [ Links ]
17. Vincent JM. A manual for the practical study of the root-nodule bacteria. A manual for the practical study of the root-nodule bacteria. 1970; [ Links ]
18. IUSS Working Group WRB. International soil classification system for naming soils and creating legends for soil maps. Food and Agriculture Organization of the United Nations. 2014;181. [ Links ]
19. Nápoles MC, Cabrera JC, Wegria G, Onderwater R, Wattiez R, Hernández I, et al. Inducción de señales en la interacción Mesorhizobium cicerii-Cicer arietinum L. Cultivos Tropicales. 2018;39(2):101-7. [ Links ]
20. Bourassa DV, Kannenberg EL, Sherrier DJ, Buhr RJ, Carlson RW. The lipopolysaccharide lipid a long-chain fatty acid is important for Rhizobium leguminosarum growth and stress adaptation in free-living and nodule environments. Molecular Plant-Microbe Interactions. 2017;30(2):161-75. [ Links ]
21. Brechenmacher L, Lei Z, Libault M, Findley S, Sugawara M, Sadowsky MJ, et al. Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. Plant Physiology. 2010;153(4):1808-22. [ Links ]
22. Kachroo A, Kachroo P. Fatty acid-derived signals in plant defense. Annual review of phytopathology. 2009;47:153-76. [ Links ]
23. Estévez J, Soria-Díaz ME, De Córdoba FF, Morón B, Manyani H, Gil A, et al. Different and new Nod factors produced by Rhizobium tropici CIAT899 following Na+ stress. FEMS microbiology letters. 2009;293(2):220-31. [ Links ]
24. Subramanian S, Smith DL. A proteomics approach to study soybean and its symbiont Bradyrhizobium japonicum-a review. In: A comprehensive survey of international soybean research-genetics, physiology, agronomy and nitrogen relationships. Londres, Reino Unido: InTechOpen; 2013. p. 978-953. [ Links ]
25. Mabrouk Y, Hemissi I, Salem IB, Mejri S, Saidi M, Belhadj O. Potential of rhizobia in improving nitrogen fixation and yields of legumes. Symbiosis. 2018;107:73495. [ Links ]
26. Franke AC, Van den Brand GJ, Vanlauwe B, Giller KE. Sustainable intensification through rotations with grain legumes in Sub-Saharan Africa: A review. Agriculture, ecosystems & environment. 2018;261(1):172-85. [ Links ]
27. Li J, Liu K, Zhang J, Huang L, Coulter JA, Woodburn T, et al. Soil-plant indices help explain legume response to crop rotation in a semiarid environment. Frontiers in plant science. 2018;9:1488. [ Links ]
28. Rahman MM, Islam AM, Azirun SM, Boyce AN. Tropical legume crop rotation and nitrogen fertilizer effects on agronomic and nitrogen efficiency of rice. The Scientific World Journal. 2014;2014:11. [ Links ]
29. Stagnari F, Maggio A, Galieni A, Pisante M. Multiple benefits of legumes for agriculture sustainability: an overview. Chemical and Biological Technologies in Agriculture. 2017;4(1):1-13. [ Links ]
30. Lötjönen S, Ollikainen M. Does crop rotation with legumes provide an efficient means to reduce nutrient loads and GHG emissions? Review of agricultural, food and environmental studies. 2017;98(4):283-312. [ Links ]
31. Waiboonya P, Elliott S. Sowing time and direct seeding success of native tree species for restoring tropical forest ecosystems in northern Thailand. New Forests. 2020;51(1):81-99. [ Links ]
32. Ali A, Erenstein O, Rahut DB. Impact of direct rice-sowing technology on rice producers' earnings: Empirical evidence from Pakistan. Development Studies Research. An Open Access Journal. 2014;1(1):244-54. [ Links ]
33. Ileri O, CARPICI EB, Erbeyi B, Suleyman A, Ali KOC. Effect of sowing methods on silage yield and quality of some corn cultivars grown in second crop season under irrigated condition of Central Anatolia, Turkey. Turkish Journal of Field Crops. 2018;23(1):72-9. [ Links ]
34. Nápoles G, Cabrera JC, Luyten E, Dombrecht B, Vanderleyden J, Hormaza J, et al. Nod factor detection in B. elkanii ICA 8001. Culture medium influence. Cultivos Tropicales. 2014;35(1):92-9. [ Links ]
35. Costales D, Nápoles MC, Falcón-Rodríguez A, González-Anta G, Petit C, Sola S, et al. Effect of chitosan polymer and inoculated with B. japonicum on soybean germination survival of seedling, nodulation and bacteria viability on seeds. Legume Research-An International Journal. 2019;42(2):265-9. [ Links ]
36. Duzan HM, Mabood F, Zhou X, Souleimanov A, Smith DL. Nod factor induces soybean resistance to powdery mildew. Plant Physiology and Biochemistry. 2005;43(10-11):1022-30. [ Links ]
37. Ganesan S, Vadivel K, Jayaraman J. Sustainable crop disease management using natural products. Boston, MA: CABI; 2015. 424 p. [ Links ]
38. Nakagawa T, Kaku H, Shimoda Y, Sugiyama A, Shimamura M, Takanashi K, et al. From defense to symbiosis: limited alterations in the kinase domain of LysM receptor-like kinases are crucial for evolution of legume-Rhizobium symbiosis. The Plant Journal. 2011;65(2):169-80. [ Links ]
Received: December 13, 2019; Accepted: March 17, 2021











 text in
text in 



