Cultivos Tropicales
ISSN 1819-4087
30--2021
Original article
Biocontrol of coffee yellow rust (Hemileia vastatrix Berk. & Br.) with Trichoderma sp. endophyte strains
1Facultad de Ciencias Agrarias, Escuela Profesional de Ingeniería Agronómica, Universidad Nacional del Altiplano, av. Floral 1153, Puno-Perú, 21001, Puno, Perú
2Escuela Profesional de Agronómica, Universidad Nacional de Cañete, Lima, Perú
3Escuela de Postgrado, Universidad Nacional del Altiplano, Puno, Perú
Yellow rust, caused by the fungus Hemileia vastatrix Berk. & Br., is one of the main diseases that limits commercial production and significantly reduces yields of coffee (Coffea arabica L.); fungicides are commonly used to control it, but the use of endophytic microorganisms such as the fungus Trichoderma is a promising alternative. Therefore, the biocontrol capacity of five endophytic Trichoderma sp. strains (TE1, TE2, TE3, TE4, TE5) on yellow rust and their effect on coffee seedling growth was determined at the nursery level. Conidial suspensions (1x107 cfu mL-1) of strains were sprayed on soil and foliage. Plant height (PH); stem diameter (SD); length of main root (LMR); number of leaves (NL); disease incidence and severity; and area under the disease progress curve (AUDPC) were evaluated. The TE-1 treatment was the most efficient in reducing disease incidence (35.8 %) and severity (8.95 %). It also improved plant growth parameters in PH (12.70 cm), SD (2.5 mm), NL (7.6 units), LMR (11.38 cm), as well as AUDPC (56.625 units), compared to the control with values of 96.67, 39.50 %, 10.02 cm, 1.96 mm, 5.06 cm, 4 units and 365.00 units in the evaluated variables, respectively. This strain could be used to improve plant development and protect against coffee yellow rust.
Key words: biological control; coffee; fungi; antagonism; pucciniales
INTRODUCTION
Coffee (Coffea arabica L.) is Peru's main agricultural export product, with great economic and social importance that generates sources of income for producers in San Juan del Oro district, Sandia province and Puno Region, with climatic conditions that favor organic coffee production. Year 2014 was the most critical one for national production. At the end of 2012 the "yellow rust" disease affected coffee´s leaves of the tree causing severe defoliation and added to the lack of renewal of old coffee plantations, brought as a consequence the decline of national production with a production of 209 182 tons 1. At world level, this disease is the main pathological problem in coffee cultivation, it can cause yield losses of up to 35 % and have a polycyclic epidemiological impact in subsequent years 2. Two approaches have been proposed to avoid losses caused by the disease. The first consists of fungicide use, which is a very costly alternative and potentially harmful to the environment. The second, through the development of improved varieties, which requires knowledge of resistance sources and pathogen diversity 3. However, the solution may have been developing for centuries in a natural way, from the interactions between organisms, such is the case of endophytic fungi that live inside the tissues of living plants without causing disease symptoms, providing ecological benefits to their host and diverse antagonistic mechanisms against pests, becoming an alternative for the biological control of diseases 4,5.
Likewise, these antagonistic endophytic fungi influence plant growth, generating resistance to biotic and abiotic stress, reflecting in plant vigor and with potential protection against the attack of pathogens; being the genus Trichoderma one of the endophytes widely studied in biological control 6-8. These fungi produce secondary metabolites and some antifungal and antibacterial compounds that inhibit the growth of other microorganisms, including plant pathogens, since they produce and release lytic enzymes that can hydrolyze a wide variety of polymeric compounds of the pathogen cell wall such as chitin, protein, cellulose and hemicellulose 5,9. In addition, the use of Trichoderma in plant disease control increases crop production for the benefit of sustainable agriculture 10.
This situation led to carry out the present research work, with the aim of determining the biocontrol capacity of Trichoderma endophyte strains towards yellow rust (Hemileia vastatrix Berk. & Br.) and its effect on the growth of coffee (Coffea arabica L.) seedlings.
MATERIALS AND METHODS
Location
The experiment was conducted in the phytopathology laboratory of the Universidad Nacional del Altiplano Puno (UNAP) and in a nursery located in San Juan del Oro district, Sandía province, Region Puno- Peru at an altitude of 1,298 m, 14° 14′ 03″ S and 69° 9′ 29″ W.
Coffee (Coffea arabica L.) seedling production
Previously, coffee seeds, Caturra variety, were germinated in beds with solarized fine sand. After 80 days, they were transplanted in 2 L polyethylene bags with solarized substrate (agricultural soil and ash) and irrigated at field capacity for four months.
Provenance and multiplication of Trichoderma strains
Five endophytic fungi strains of the genus Trichoderma sp. were provided by the Phytopathology laboratory of the Universidad Nacional del Altiplano-Puno. These were isolated from leaves (strains TE1 and TE2) and stems (strains TE3, TE4 and TE5) of coffee plants var. Catimor from San Juan del Oro district in Papa dextrose Agar medium and conserved in a 20 % glycerin solution at -5 °C. For multiplication, strains were reactivated in Petri plates with PDA medium, half a plate with the fungus was deposited in polypropylene bags with solid substrate, sterilized with pre-cooked barley, incubated at 25 °C and removed for 15 days until drying, these were harvested and conserved in sealed polypropylene bags at 5 °C 11,12.
Trichoderma application
Previously, the spore count of the barley substrate was carried out by serial dilutions, and then a spore suspension of 1x107 cfu mL-1 was obtained in sterile distilled water. Three applications were made to the substrate and one to the foliage in coffee stands. The first application was at time of transplanting the seedlings, and then the second and third applications were made every thirty days, spraying the substrate with 200 mL of the suspension 8. Thirty days after the third application, foliar spraying made growth parameters were evaluated in plants three months after transplanting, then a fourth application.
Inoculation of Hemileia vastatrix Berk. & Br
For the inoculum, leaves with presence of yellow rust pustules were collected from coffee cultivation fields of Caturra variety, pieces of leaves (0.5 cm2) with disease signs were obtained, these were submerged in sterile distilled water with tween 80 to detach the uredospores from the tissue and were standardized to a concentration of 2x104 uredospores mL-1 (13,14. Inoculation was carried out by spray inoculation directed to the foliage of 94-day-old coffee seedlings after transplanting (dat). After 24 hours, Trichoderma sp. endophyte strains were applied at a concentration of 1x107 cfu mL-1.
Parameter evaluations
At three months of age after replanting, five plants were evaluated for each treatment; for plant height (cm), measurements were taken from the plant collar to the stem apex. For stem diameter (mm) at a height of 3 cm from the ground; for main root length (cm) from plant collar to the main root cap; and for the number of leaves (unit), the total number of leaves per plant was counted.
After 23 days of pathogen inoculation, five evaluations were made every five days. For incidence, the number of leaves with the presence of the disease and the number of leaves observed per coffee plant posture were evaluated. For severity, two leaves per plant were evaluated with the help of a scale 15, with these evaluations the AUDPC (area under the disease progress curve) was determined.
Statistical analysis
To evaluate the effect of Trichoderma sp endophyte strains on the growth of coffee seedlings and the biocontrol capacity towards yellow rust (Hemileia vastatrix Berk. & Br.), a Completely Randomized Design was used, with five strains of Trichoderma and two controls (sick and healthy) making a total of seven treatments with five repetitions. The data expressed in percentage were transformed to log, with whose transformation the normality and the homogeneity of variances were confirmed, then the analysis of variance (ANOVA) and Duncan contrast tests were carried out, with a confidence level of 95 % and a margin of error of 5 %, with the use of a statistical software InfoStat, version 2008.
RESULTS
Effect of native Trichoderma sp endophytic strains on the growth of coffee (Coffea arabica L.) seedlings
Nine weeks after the transplanting of the coffee seedlings, the height of the plant and the main root increased significantly, in the presence of all the strains of endophytic Trichoderma, in comparison with the control (without Trichoderma application). However, in the stem diameter, the treatment TE3 did not show significant difference, in relation to the control without application, as well as in treatments TE4 and TE5 in number of leaves. It is necessary to emphasize that in these treatments strains from coffee stems were used. However, treatments TE1 and TE2 are strains from coffee leaves that significantly increased plant height, main root and number of leaves. Meanwhile, in stem diameter, treatment TE1 had the best effect, followed by TE5, TE2 and TE4 with no significant difference with respect to the control (Table 1). The treatment applied with strain TE1 showed the greatest increase in plant height, stem diameter, main root and number of leaves with 21.10, 21.60, 55.54 and 47.34 %, respectively, and compared to the control.
Table 1 Growth of coffee (Coffea arabica L.) seedlings treated with Trichoderma sp. endophyte strains, under nursery conditions in San Juan del Oro district
| Treatments (Strains) | Plant height (cm) | Stem diameter (mm) | Main root (cm) | Leaf (unit) |
|---|---|---|---|---|
| TE1 | 12.70 a | 2.50 a | 11.38 a | 7.60 a |
| TE2 | 12.10 ab | 2.27 b | 9.50 b | 7.20 ab |
| TE3 | 10.94 c | 2.12 bc | 6.58 c | 4.80 bc |
| TE4 | 11.42 bc | 2.16 b | 6.48 d | 5.20 cd |
| TE5 | 11.64 b | 2.25 b | 8.10 d | 6.00 cd |
| Contrl | 10.02 d | 1.96 c | 5.06 e | 4.00 d |
| CV | 5.35 % | 6.028 % | 8.23 % | 10.64 % |
| R2 | 0.710 | 0.651 | 0.929 | 0.652 |
According to Duncan's test, means with different letters indicate significant differences (p≤0.05)
Each treatment had five replicates. Control: without Trichoderma application
Biocontrol capacity of Trichoderma sp. on yellow rust (Hemileia vastatrix Berk. & Br.)
In five evaluations of yellow rust incidence in coffee seedlings, at the nursery level, there were significant differences among treatments (p≤0.05). At 117 days after transplanting (dat), disease symptoms were observed, such as chlorotic spots on the upper side of the leaves without the presence of pustules. In this first evaluation the diseased control presented a high incidence, followed by the treatments with Trichoderma applications TE3, TE4 and TE5; however, in the treatments TE1 and TE2 presented low incidence. In the second, third and fourth evaluation of incidence, treatments TE3, TE4 and TE5 did not show significant differences with the diseased control. However, in the fifth evaluation, treatments TE4 and TE5 did show significant differences with the diseased control. Therefore, for all the evaluations, TE1 and TE2 were the best bio-controllers because they presented lower incidence percentages, unlike treatments TE3, TE4 and TE5, which were not efficient in reducing the disease (Table 2).
Table 2 Incidence of yellow rust (Hemileia vastatrix Berk. & Br.) in coffee seedlings treated with Trichoderma sp. endophyte strains under nursery conditions in San Juan del Oro district
| Treatments | Incidence evaluations (%) | ||||
|---|---|---|---|---|---|
| 1rst (117 dat) | 2nd (122 dat) | 3rd (127 dat) | 4th (132 dat) | 5th (137 dat) | |
| TE1 | 3.33 b | 5 bc | 17.5 c | 24.99 b | 35.8 c |
| TE2 | 3.33 bc | 10 b | 22.5 bc | 27.50 b | 42.5 c |
| TE3 | 10 b | 28.33 a | 40.83 a | 61.67 a | 73.33 ab |
| TE4 | 12.5 b | 31.67 a | 43.33 a | 56.67 a | 70.00 b |
| TE5 | 11.67 b | 26.67 a | 35.83 ab | 53.33 a | 64.17 b |
| C-dis. | 36.67 a | 50 a | 65.00 a | 86.66 a | 96.67 a |
| C-hea | 0 c | 0 c | 0 d | 0 c | 0 d |
According to Duncan's test, means with different letters indicate significant differences (p≤0.05).
Each treatment had five replicates. C- dis: disease control (with pathogen inoculation)
C-hea: healthy control (without pathogen inoculation). dat: days after transplanting.
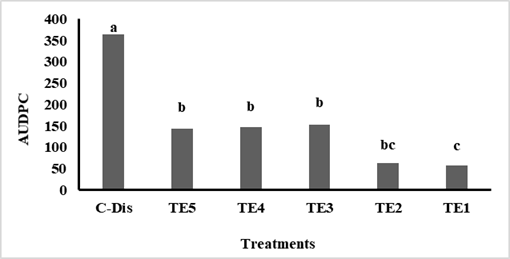
With respect to the severity (%), in the five evaluations all the treatments that received Trichoderma sp. applications reduced the disease significantly with respect to the diseased control (C-dis) that was the most affected. The coffee stands treated with treatments TE1 and T2 were the least affected by the disease with lower severity values of 10.30 % up to the 5th evaluation (Table 3). Similarly, these treatments had the lowest AUDPC values of 56.63 and 63.38, respectively, with respect to the other treatments and the diseased control with 365 units (Figure 1).
Table 3 Percentage of severity (%) of yellow rust (Hemileia vastatrix Berk. & Br.) of coffee seedlings treated with Trichoderma sp. strains under nursery conditions
| Treatments | Number of severity assessments dat | ||||
|---|---|---|---|---|---|
| 1rst (117 dat) | 2nd (122 dat) | 3rd (127 dat) | 4th (132 dat) | 5th (137 dat) | |
| TE1 | 0.3 cd | 1.2 c | 1.2 c | 4.1 d | 8.95 c |
| TE2 | 0.3 cd | 1.2 c | 1.5 c | 4.7 cd | 10.3 c |
| TE3 | 1.5 bc | 2.1 b | 4.7 b | 11.25 bc | 23.3 b |
| TE4 | 1.2 bc | 2.7 b | 5 b | 10.25 bc | 22 b |
| TE5 | 1.2 bc | 2.7 b | 6 b | 12.25 b | 14.3 bc |
| C-dis. | 3 a | 10 a | 15.3 a | 26.5 a | 39.5 a |
| C-hea | 0 d | 0 d | 0 c | 0 e | 0 d |
According to Duncan's test, means with different letters indicate significant differences (p≤0.05).
Mean severity data for five plants per treatment. C- dis: disease control (with pathogen inoculation).
C-hea: healthy control (without pathogen inoculation). dat: days after transplanting.
DISCUSSION
In the investigation, there was a positive effect on coffee growth (Coffea arabica L.) seedlings at nursery level with the application of endophytic Trichoderma sp strains from leaves and stems of coffee plants. Similar results were reported by other authors, who point out the increase in plant size and the number of leaves in coffee seedlings at nursery level, with applications of Trichoderma sp. endophyte coming from coffee plants of Catimor variety 16. On the other hand, with the T. harzianum applications in coffee seedlings, a greater root length, plant height, stem diameter and number of leaves were obtained 17,18. Similarly, in other crops such as cocoa, plant growth was promoted at the pot level, the number of leaves, plant height, shoots and root dry matter increased significantly 19, yield improved in the cultivation of quinine and grapevine 20,21 and the number of leaves increased in rice 12. Possibly this is due to the capacity of these Trichoderma strains to acidify the rhizosphere, releasing organic acids and chelating metabolites that sequester cations and redox activity; these mechanisms make minerals soluble 22. On the other hand, it is known that Trichoderma species are potential root colonizers, produce auxins, cytokinins and ethylene that are involved in the growth and protection of plants against pathogen attack 23,24, and induce resistance to diseases in a variety of plant species 6.
Likewise, the native Trichoderma sp. endophyte strains used in this research had the capacity to control the pathogen H. vastatrix, reducing the incidence, severity and AUDPC of the disease in coffee seedlings, since they were treated preventively with the strains at the root system level he and after the inoculation of the pathogen to the foliage. On the other hand, strains from leaves were the ones that presented the best effect in reducing the disease, because these endophytes can produce an antifungal compound or a substance that can induce the defense mechanisms of the plant against the pathogen 6. In the same way, provoke systemic resistance induced by pathways dependent on jasmonic acid/ethylene and trigger defense responses in the plant because of the establishment of some Trichoderma strains in the rhizosphere 25.
Other mechanisms used by Trichoderma are the physical competition for space and nutrients; production of secondary metabolites with antibiotic or antifungal activity; mycoparasitism secreting hydrolytic enzymes like chitinases and glucanases that degrade the cellular wall of the phytopathogenic fungi 26-28. This agrees with the reported by other authors, who confirm antagonism of Trichoderma strains on Hemileia vastatrix Berk. & Br. because of the Trichoderma colonization on the pathogen and by competition for space and nutrients on coffee leaves with symptoms of the disease 29; likewise, these antagonists induce resistance in the plant to infections by the pathogen 6. Likewise, they affirm mycoparasitism of Trichoderma strains on Phytophthora megakarya, where these antagonists significantly reduced the pathogen effects on plant leaves 9. At the same time, in preliminary studies, they indicate that strains of Trichoderma endophytes that were isolated from Coffea flowers in Africa inhibit the germination of uredospores of H. vastatrix and reduce the severity of the disease. It is considered as a potential biological controller for the development of biofungicides, because Trichoderma sp. possesses different characteristics such as protecting plants against root pathogens 30. For all the mechanisms of action that the Trichodema strains possess and also for being coffee endophyte, the results obtained in the investigation are explained, about that all the Trichoderma endophyte strains had effect in the control of yellow rust in coffee plants of four months of age.
CONCLUSION
It was demonstrated that with applications of Trichoderma endophyte strains to the soil and foliage in coffee seedlings, the incidence and severity of yellow rust (H. vastatrix), and the growth parameters of the seedlings at nursery level are improved. The strain TE1, coming from coffee leaves is the one with the best response in the growth parameters (plant height, stem diameter, main root length and number of leaves); also with lower percentage of incidence, severity and AUDPC, in comparison with the control without Trichoderma application.
BIBLIOGRAFÍA
1. MINAGRI. Síntesis agroeconómica del café [Internet]. Perú: Ministerio de Agricultura y Riego; 2015 Jun [cited 18/02/2021] p. 15. Report No.: 1. Available from: https://bibliotecavirtual.midagri.gob.pe/index.php/analisis-economicos/estudios/2015-1/31-sintesis-agroeconomica-del-cafe/file1. [ Links ]
2. Talhinhas P, Batista D, Diniz I, Vieira A, Silva DN, Loureiro A, et al. The coffee leaf rust pathogen Hemileia vastatrix: one and a half centuries around the tropics. Molecular Plant Pathology. 2017;18(8):1039-51. doi:10.1111/mpp.12512 [ Links ]
3. Quispe-Apaza CS, Mansilla-Samaniego RC, López-Bonilla CF, Espejo-Joya R, Villanueva-Caceda J, Monzón C. Diversidad genética de Hemileia vastatrix de dos zonas productoras de café en el Perú. Revista mexicana de fitopatología. 2017;35:418-36. doi:10.18781/R.MEX.FIT.1612-7 [ Links ]
4. Martinez NLM, Martínez IJE, Rodríguez EQ, Heil M. El papel de los volátiles en la delimitación del espacio de hongos endófitos de frijol Lima. Jovenes en la Ciencia. 2018;3(0):357-61. [ Links ]
5. Vásquez MV, Lozano RE, Martínez SP, Castillo DS del. Hongos endófitos foliares como candidatos a biocontroladores contra Moniliophthora spp. de Theobroma cacao (Malvaceae) en Ecuador. Acta Biológica Colombiana. 2018;23(3):235-41. doi:10.15446/abc.v23n3.69455 [ Links ]
6. Bisen K, Keswani C, Patel J, Sarma B, Singh H. Trichoderma spp.: efficient inducers of systemic resistance in plants. In: Microbial-mediated induced systemic resistance in plants [Internet]. Springer, Singapur; 2016. p. 185-95. Available from: https://link.springer.com/chapter/10.1007/978-981-10-0388-2_126. [ Links ]
7. Contreras-Cornejo HA, Macías-Rodríguez L, del-Val E, Larsen J. The root endophytic fungus Trichoderma atroviride induces foliar herbivory resistance in maize plants. Applied Soil Ecology. 2018;124:45-53. [ Links ]
8. Leon-Ttacca B, Arévalo-Gardini E, Bouchon AS. Muerte repentina de Theobroma cacao L. causado por Verticillium dahliae Kleb. en el Perú y su biocontrol In vitro. Ciencia y Tecnología Agropecuaria [Internet]. 2019 [cited 30/04/2020];20(1). doi:10.21930/rcta.vol20_num1_art:1251 [ Links ]
9. Saravanakumar K, Fan L, Fu K, Yu C, Wang M, Xia H, et al. Cellulase from Trichoderma harzianum interacts with roots and triggers induced systemic resistance to foliar disease in maize. Scientific reports. 2016;6:35543. [ Links ]
10. Al-Ani LKT. Trichoderma: beneficial role in sustainable agriculture by plant disease management. In: Plant Microbiome: Stress Response. Springer; 2018. p. 105-26. [ Links ]
11. Sandoval MC, Belesansky C. Producción artesanal del hongo antagónico Trichoderma Persoon en sustrato sólido. 2020;7(3):55-64. [ Links ]
12. Chávez Vergara JAA, Torres García CA, Espinoza Vera EA, Zambrano Pazmiño DE, Villafuerte Barreto AG, Zambrano Gavilanes FE, et al. Efectos de la cepa nativa de Trichoderma sp. y lixiviado de vermicompost bovino sobre el crecimiento foliar y contenido de clorofila en arroz (Oryza sativa L.) en fase de semillero. Ecuador. 2020;7(2):23-31. doi:10.36331/revista.v7i2.104 [ Links ]
13. García EG, Jiménez E, Castro O, Mora B. Variación en la composición química foliar de Coffea sp. (Rubiales: Rubiaceae) y su. relación con la resistencia a Hemileia vastatrix (Uredinales: Pucciniaceae). Revista de biología tropical. 1993;209-14. [ Links ]
14. Cruz IG-DL, Perez-Portilla E, Escamilla-Prado E, Martínez-Bolaños M, Carrión-Villarnovo GLL, Hernández-Leal TI. Selección in vitro de micoparásitos con potencial de control biológico sobre Roya del café (Hemileia vastatrix). Revista Mexicana de Fitopatología [Internet]. 2017;36(1). Available from: https://www.smf.org.mx/rmf/ojs/index.php/RMF/article/view/9314. [ Links ]
15. SENASA. Norma para la ejecución y remisión de información de actividades del programa manejo integrado de plagas del cafeto [Internet]. Lima. Perú; 2003. Available from: https://www.senasa.gob.pe/senasa/descargasarchivos/jer/SUB_DIR_CONTEP/1222.pdf15. [ Links ]
16. Alarcon C, Shamir F. Identificación de hongos endófitos y su uso en la bioproteccion de plántulas de café para reducir el daño de Colletotrichum coffeanum en San Martin-Perú [Internet] [Pregrado - Agronomia]. [Tarapoto, Peru]: Universidad Nacional de San Martin; 2017. 58 p. Available from: http://repositorio.unsm.edu.pe/handle/11458/241816. [ Links ]
17. Guilcapi Pacheco ED. Efecto de Trichoderma harzianum y Trichoderma viride, en la producción de plantas de café (Coffea arabica) variedad caturra a nivel de vivero [Internet] [Pregrado - Agronomia]. [Riobamba, Ecuador]: Escuela Superior Politécnica de Chimborazo; 2010. 95 p. Available from: http://dspace.espoch.edu.ec/handle/123456789/33417. [ Links ]
18. Borja Espinoza JM, Rivera Meza A. Influencia del hongo Trichoderma harzianum en la producción de plantas de café (Coffea. arabicavar. laurina [Smeathman], caturra) [Internet] [Tesis de pregrado]. [La Merced- Perú]: Universidad Nacional Daniel Alcides Carrión; 2018 [cited 17/03/2021]. Available from: http://repositorio.undac.edu.pe/handle/undac/210318. [ Links ]
19. Tchameni SN, Ngonkeu M, Begoude B, Nana LW, Fokom R, Owona A, et al. Effect of Trichoderma asperellum and arbuscular mycorrhizal fungi on cacao growth and resistance against black pod disease. Crop protection. 2011;30(10):1321-7. [ Links ]
20. Leon Ttacca B, Ortiz Calcina N, Condori Ticona N, Chura Yupanqui E. Cepas de Trichoderma con capacidad endofitica sobre el control del mildiu (Peronospora variabilis Gäum.) y mejora del rendimiento de quinua. Revista de Investigaciones Altoandinas. 2018;20(1):19-30. doi:10.18271/ria.2018.327 [ Links ]
21. Pascale A, Vinale F, Manganiello G, Nigro M, Lanzuise S, Ruocco M, et al. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop protection. 2017;92:176-81. doi:10.1016/j.cropro.2016.11.010 [ Links ]
22. Altomare C, Norvell WA, Björkman T, Harman GE. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Applied and environmental microbiology. 1999;65(7):2926-33. [ Links ]
23. Castro A, Rivilla C. Trichoderma spp modos de acción eficacia y usos en el cultivo de café [Internet]. Caldas, Colombia; 2012. Available from: https://biblioteca.cenicafe.org/handle/10778/57723. [ Links ]
24. Acurio Vásconez RD, España Imbaquingo CK. Aislamiento, caracterización y evaluación de trichoderma spp. como promotor de crecimiento vegetal en pasturas de raygrass (Lolium perenne) y Trébol blanco (trifolium repens). La Granja: Revista de ciencias de la vida. 2017;25(1):53-61. doi:10.17163/lgr.n25.2017.05 [ Links ]
25. Salas-Marina MA, Silva-Flores MA, Uresti-Rivera EE, Castro-Longoria E, Herrera-Estrella A, Casas-Flores S. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. European Journal of Plant Pathology. 2011;131(1):15-26. [ Links ]
26. Bae SJ, Mohanta TK, Chung JY, Ryu M, Park G, Shim S, et al. Trichoderma metabolites as biological control agents against Phytophthora pathogens. Biological Control. 2016;92:128-38. doi:10.1016/j.biocontrol.2015.10.005 [ Links ]
27. González BC, Arizmendi GD, Velasco RG. Trichoderma: su potencial en el desarrollo sostenible de la agricultura. Biotecnología Vegetal. 2019;19(4):237-48. [ Links ]
28. Singh A, Shukla N, Kabadwal B, Tewari A, Kumar J. Review on plant-Trichoderma-pathogen interaction. International Journal of Current Microbiology and Applied Sciences. 2018;7(2):2382-97. doi:10.20546/ijcmas.2018.702.291 [ Links ]
29. Rolz A, De León L, Paniagua O. Evidencia de un antagonismo in vitro de especies de Trichoderma contra Hemileia vastatrix (roya del café). Centro de Ingeniería Bioquímica, Instituto de Investigaciones. Universidad del Valle de Guatemala. Revista 25 de la Universidad del Valle de Guatemala. 2013;25:61-5. [ Links ]
30. Barreto RW, Augustín CAA, Herrera RM, Salcedo SS, Evans H. Controle biológico da ferrugem do cafeeiro com fungos micoparasitas e endofíticos. In: Viçosa UF de, editor: Universidad Federal de Viçosa, Viçosa, MG, Brasil; 2017. Available from: http://www.infobibos.com/anais/CBFito/50/Resumos/Resumo50CBFito_0011.pdf30. [ Links ]
Received: May 16, 2020; Accepted: June 10, 2021