Cultivos Tropicales
ISSN 1819-4087
30--2021
Original article
Some physical, chemical and microbiological properties of an agricultural soil in Darien, Republic of Panama
1Universidad de Panamá, Centro Regional Universitario de Darién, CRUD, Corregimiento de Metetí, Distrito de Pinogana, Comunidad de Villa Darién, Estafeta Universitaria Apartado 3366, Panamá
2Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
3Centro de Biodiversidad y Descubrimiento de Drogas, Instituto de Investigaciones Científicas y Servicios de Alta Tecnología (INDICASAT), Apartado Postal 0843-01103. Edificio 219, Ciudad del Saber, Clayton Panamá, República de Panamá
For any study of soils it is always necessary a previous profile description, in which one observes their morphology and they are presented a series of estates that they are the result of the soil and the antropogenic influence formation. Taking in consideration this premise, it was carried out the soil profile description in a farm of Yaviza, Panama, and to determine some of their physical, chemical and microbiological properties. The soil was classified as Vertic Gleysol, due to the found diagnosis characteristics: gleyic properties to less than 50 cm of depth and vertic properties, the soil have clayey texture, it was not compacted and the content of organic matter and the organic carbon reserve of the soil were low. The pH was acid in the whole profile, however, the effective capacity of cationic exchange oscillated between high and half, with prevalence of the ions Ca2+ and Mg2+ and a high saturation percentage of bases. Except the relationship Ca/Mg that was normal, the other ones internutrients relate were outside of range. Microelement concentrations and changeable aluminum were low in the whole profile. When analyzing the relative abundance of microorganisms it was found that the most abundant phylum were Actinobacteria, Proteobacteria, Firmicutes and Acidobacteria, all of the domain Bacteria and of the Kingdom Fungi the prevalence of the phylum Ascomycota was observed, followed by Basidiomycota and Rozellomycota. It is necessary to correct the imbalance between the nutrients and check the evolution of these estates during the agricultural exploitation of the area in study.
Key words: Gleysol; morphological characteristics; fertility; soil microorganisms
INTRODUCTION
Soil is an important natural resource with great influence on the environment and the economy. To a large extent, the survival and well-being of the present population and future generations depend on it 1. Soil fertility is considered a determining factor in the availability of nutrients for cultivated plants. It is difficult to understand the nutrition physiology of economic crops without an adequate study of indicators that shape the fertility and nature of soils, especially in tropical regions 2.
However, in soil fertility studies it is indispensable to include analyses related to soil biology because most of the nutrient transformations in the soil are carried out by soil microorganisms. In this regard, several studies have established the importance of soil microbiota and their interactions for nutrient mineralization and plant nutrition 3.
For any soil study, a previous description of the soil as such is always necessary. On the one hand, it is the first contact with soils that may exist in the study region and, on the other hand, the soil profile is manifested through its morphology, which presents a series of properties that are the result of soil formation and anthropogenic influence, if any 4.
The initial analysis of the physical, chemical and biological properties of a soil is an indispensable step to proceed to a sustainable agricultural management, taking into consideration limiting factors and propitiating a management that preserves, improves and increases both soil properties and agricultural productions without affecting the environment.
Taking into consideration the importance of describing and characterizing any soil with an agricultural use, the present research was carried out with the objective of describing a soil profile and determining some of its physical, chemical and microbiological properties.
MATERIALS AND METHODS
For the characterization of some physical, chemical and microbiological properties, a soil was selected from the "El Mamey" farm, located 800 m south of the Pan-American Highway, in Yaviza town, Pinogama district, Darien province, Republic of Panama. This soil was covered with natural grasses, was subjected to fire and was under agricultural tillage for subsequent planting of annual crops.
A visual examination of the soil was made and with the help of shovels, a test pit was opened, approximately in the soil center and in the middle part of the slope. The dimensions of the trench were 1 m by 1 m in length and width and 1.12 m depth. Once the opening was made, the soil was described (4) and colors were established according to the Munsell Table 5. Soil classification was carried out following the Cuban Soil Classification 2015 6, the World Reference Base 7) and the Soil Taxonomy 8.
Subsequently, samples were taken in triplicate at the profile depth to determine the natural humidity, by the gravimetric method and bulk density, by the method of cutting cylinders 9. Triplicate samples of approximately 500 g were also taken from each horizon and taken to Instituto de Investigaciones Agropecuarias de Panamá (Panama Agricultural Research Institute) (IDIAP) for analysis of some physical and chemical properties. The results were evaluated according to the analytical methods used.
Variables evaluated and the determination methods used at IDIAP were: texture, by the Bouyoucos method, pH in water in a 1:2.5 ratio by potentiometry. The percentages of C and organic matter (OM) were determined by Walkley-Black. The N content was calculated by multiplying the percentage of OM by 0.05. Soil organic carbon stocks were calculated by the following equation 10:
where:
SOC= total soil organic carbon per surface area (Mg C ha-1).
OC= total organic carbon (%)
Da = bulk density (Mg m-1)
m = soil depth (cm)
P, K, Mn, Fe, Zn and Cu were extracted using Mehlich-1 extractant solution and determined by atomic absorption. For the Ca, Mg and Al extraction, 1N potassium chloride was used, Ca and Mg were determined by atomic absorption and Al by titration with 0.01 N NaOH 11.
The internutrient ratios were established by calculation from contents of these nutrients in the soil solution. The saturation percentage by bases was calculated from the Base Exchange Capacity divided by the Cationic Exchange Capacity and multiplied by 100. The effective Cationic Exchange Capacity (CECe) was calculated from the sum of the cations and the exchangeable acidity. The percentage of aluminum saturation was calculated from the aluminum in the soil divided by the CECe 12.
For the microbiological analysis of the soil, a simple random sampling was carried out throughout the field, taking 14 samples, each consisting of five subsamples, at 0-20 cm depth. After the corresponding identification, they were frozen at -18 ºC and then transferred in a portable cooler for processing at the Institute of Scientific Research and High Technology Services (INDICASAT).
Total genomic DNA (gDNA) extraction from soil samples was performed using the DNeasy PowerSoil Pro reagent kit (QIAGEN, Hilden, Germany), following the manufacturer's protocol. For DNA library construction, two different types of amplifications were performed for each sample using two primer combinations, one for bacteria and archaea and one for fungi. In all cases, PCR (Polymerase Chain Reaction) was performed in triplicate in a thermal cycler.
For the study of bacteria and archaea present in the soil, primers 515F (GTGCCAGCMGCCGCGGTAA) and 806R (GGACTACHVGGGGGTWTCTAAT), which amplify the V4 region of the 16S ribosomal DNA, were used. While for the study of fungal communities, primers ITS1F (CTTGGTCTCATTTAGAGGAAGTAA) and ITS4A (CGCCGTTACTGGGGGGGGCAATCCCTG) that amplify the ITS (Internal Transcribed Spacers) region of ribosomal DNA were used. The primers contained adapters for the Illumina MiSeq sequencing platform 13.
Both PCRs were run under the following conditions: 3 min at 94 °C, 35 cycles of 45 s at 94 °C, 1 min at 50 °C, and 90 s at 72 °C, followed by a final elongation of 10 min at 72 °C. In all cases reaction mixtures contained 1x PCR Buffer, 700 μM of MgCl2, 400 μM of each dNTP, 500 μM of primers, 0.45x Q solution, 2U of taq DNA polymerase (QIAGEN) and 10-30 ng gDNA in 25μL. PCR products were visualized by electrophoresis on agarose gels (1.5 %) using RedGel as developer and 1x TAE run buffer (Tris 40 mmol L-1 (pH 7.8), acetic acid and EDTA) was used. The runs were performed at 80 volts for 40 minutes in horizontal electrophoresis equipment. To corroborate the presence of the correct amplicons, the 1kb plus DNA Ladder molecular weight marker (Invitrogen) was used.
Subsequently, a second PCR was performed in all cases to add indices (SA5F and SA7R) to the sequences 13. The conditions used were: 3 min at 94 °C, 6 cycles of 45 s at 94 °C, 1 min at 50 °C and 90 s at 72 °C, followed by a final elongation of 10 min at 72 °C. The reaction mixtures contained 1x PCR Buffer, 1.5 mM MgCl2, 800 μM of each dNTP, 200 nM of the indels, 1.25 U of taq DNA polymerase (QIAGEN) and 2 μL of the PCR1 pool in 25 μL. Once the presence of the correct amplicons was verified by performing electrophoresis as previously described, the DNA library was purified using the SequalPrep™ Normalization Plate reagent set according to the manufacturer's instructions. DNA concentration was quantified using the Qubit® dsDNA BR Assay reagent set (Life Technologies), obtaining a concentration of approximately 10 ng/μl. A Bioanalyzer (Agilent 2100 Bioanalyzer) was used to determine the size, purity and concentration of the library obtained. Finally, the samples were mixed in equimolar amounts and sequenced on the Illumina MiSeq platform 13.
The sequences obtained were subjected to bioinformatic analysis as previously described 13, for which a similarity of 97 % was defined and the QIIME package was used. The OTUs (operational taxonomic units) were assigned a taxonomic identity by confronting representative sequences of each against the NCBI (National Center for Biotechnology Information of the United States) database using BLAST. The identities obtained were used to determine the distribution of sequences among the major bacterial, archaeal and fungal phyla.
RESULTS AND DISCUSSION
Soil profile description (Table 1)
Classification
Cuba: Vertic, chromic gleysol without carbonates 6. WRB 2014: Vertic, eutrophic Gleysol 7. Soil Taxonomy: Vertic Endoaquept 8
Diagnoses
Diagnostic horizon: Vertic horizon. Diagnostic characteristics: Gleyic properties at less than 50 cm depth and vertic properties.
Location
Location: In Yaviza, south of the Pan-American Highway. Municipality: Pinogana. Province: Darien. Country: Panama
Height (m a.s.l.): 31 m. Geographical coordinates: Lat N 8,203 Long W -77,74
Formation factors
1. Landform: hilly
1.1 Physiographic position of the site (position within the relief): mid-slope
1.2 Topography of the surrounding terrain (within 1 km of the test site): hilly
2. Slope where profile is taken: ≈7°
3. Vegetation or land use: in preparation (tillage), formerly natural pasture (Paspalum virgatum L.) that was burned with fire
4. Climate: Tropical sub-humid (Aw)
5. Source material: Sedimentary
6. Time: Quaternary
7. Internal drainage: poor
8. Surface drainage: regular
Table 1 Description of the soil profile and identified horizons
| Horizon | Depth (cm) | Description |
|---|---|---|
| A1(v) | 0-22 | Clay texture, color 2,5Y5/1 gray yellowish, consistent blocks prismatic structure, wet. With slicken sides. Abundant roots. Without stones and other inclusions. With ants and small spiders. No acid reaction. Gradual transition. Presence of cracks that extend in the depth of the other horizons |
| A2(v) | 22-36 | Clay texture, color 2,5Y5/1 gray yellowish, consistent blocks prismatic structure, wet. With slicken sides. Abundant roots and ants. No acid reaction. Abrupt transition |
| C1g | 36-59 | Clay texture, color 2,5Y7/6 yellowish, consistent and massive polyedric structure, wet. With slicken sides. Presence of fine roots, color stains 2,5Y7/1 light gray. No acid reaction. Gradual transition. |
| C2g | 59-112 | Clay texture, color 5Y7/6 yellow, big, consistent and massive polyedric structure, wet. Presence of some fine roots, color stains N7/0 white grayish. No acid reaction. |
The predominant clay type, identified by visual observation, is the 2:1, montmorillonite group. The characteristics that allowed identifying the presence of the clay were the block structure 14, the high moisture retention, the high plasticity, the presence of sliding faces and the presence of cracks throughout the soil, product of the shrinkage that naturally occurs in these clays in the low rainfall period 15. This type of clay has a high humidity retention, which is manifested by the gleyic properties detected at a depth of less than 50 cm, so that poor internal drainage and high humidity retention can be considered as possible limiting factors for production.
Study of some physical and chemical properties
Table 2 shows the main analytical results of the soil profile. When analyzing the mechanical composition, an increase in clay content was observed with the depth of the profile, moving the textural classification from clay loam to clayey.
Table 2 Some physical properties, mechanical composition, organic matter and soil organic carbon stocks of the four horizons identified in the profile
| Horizon/Depth (cm) | Sand (%) | Loam | Clay | Textural classification | NH (%) | Da (Mg kg-3) | OM (%) | N | C | SOC |
|---|---|---|---|---|---|---|---|---|---|---|
| (Mg C ha-1) | ||||||||||
| A1(v)/0-22 | 36 | 28 | 36 | Clay-loam | 34.35 | 0.90 | 1.14 | 0.06 | 0.66 | 13.14 |
| A2(v)/22-36 | 32 | 24 | 44 | Clay | 37.22 | 1.12 | 0.21 | 0.01 | 0.12 | 1.92 |
| C1g/36-59 | 20 | 24 | 56 | Clay | 43.69 | 0.98 | 0.04 | 0.00 | 0.02 | 0.52 |
| C2g/59-112 | 8 | 20 | 72 | Clay | 38.35 | 1.01 | 0.00 | |||
NH: natural humidity. Da: soil density. OM: soil organic matter. SOC: soil organic carbon reserve
With regard to this result, during the gleyzation process there is a very strong change in the composition and properties of the mineral part of the soil, because it is affected by different complex transformations, the destruction of primary and secondary minerals takes place and at the same time the synthesis of secondary minerals of neoformation (clays) occurs; that is, during the process of gleyzation, there is formation of clays; therefore, in most cases, the gleyzed horizon has a more clayey mechanical composition than those that are not 16.
The natural humidity ranged between 34 and 43 %, which is an adequate range, if it is taken into account that the samplings were made at the end of the low rainfall period. This high humidity is in correspondence with the type of soil (vertic Gleysol), with the presence of clays of the montmorillonite group, with high humidity retention 14.
The bulk density was low throughout the profile, so it can be assumed that the soil was not compacted. This result corresponds to the presence of natural grasses. The quantity of roots of plants such as Paspalum virgatum and the depth to which they reach during their growth cause good soil aggregation, porosity and low bulk density, which creates excellent physical conditions for crop development 17. Bulk density and root growth have a high and positive correlation 18; that is, with a low bulk density there is no impediment to root growth in the profile, an aspect that was observed in the description of this soil profile, in which the presence of roots was found even at the greatest depth (59-112 cm). In addition, soils with vegetation cover or those that have not been subjected to high tillage pressures have low bulk density or bulk density values 19.
Organic matter values and C and N percentages were low throughout the profile. The percentage of C in the first horizon is lower than previously reported 19, below which the soil is considered to be degraded. It is possible that these low values are due to the fact that prior to sampling, the site was burned with fire and later plowing was started to carry out soil tillage and it has been shown that conventional burning and plowing of the soil with prism inversion tends to decrease organic matter contents and carbon stocks in the profile 10.
Fire changes the dynamics of the C cycle in the soil, altering physical, chemical and biological properties, thus reducing the content and composition of organic matter and the availability of N and P, at least in the first months after the fire 20.
Table 3 shows the chemical variables evaluated in the soil profile. The pH was classified as acid throughout the profile, except in the 22-36 cm depth, which was classified as slightly acidic. Ca and Mg contents were high throughout the depth, while P was low. However, K was medium at the 0-22 cm and 36-59 cm depths, while it was low at the others. Saturation percentage by bases was high throughout the profile and the effective cation exchange capacity was high at the depths of 0-22 cm and 59-112 cm and medium at the remaining depths.
Table 3 Main chemical properties of four horizons identified in the profile
| Horizon/Depth | pH | Ca | Mg | K | P | Base saturation | CECe |
|---|---|---|---|---|---|---|---|
| (cm) | (H2O) | (cmolc kg-1) | (mg L-1) | (%) | (cmolc kg-1) | ||
| A1(v)/0-22 | 5.90 | 34.00 | 6.50 | 61.30 | 1.00 | 99.75 | 40.76 |
| A2(v)/22-36 | 6.30 | 27.90 | 7.40 | 38.20 | 1.00 | 99.72 | 35.50 |
| C1g/36-59 | 5.40 | 22.40 | 9.50 | 46.60 | 0.00 | 92.49 | 34.62 |
| C2g/59-112 | 5.80 | 37.20 | 17.20 | 30.60 | 0.00 | 98.73 | 55.18 |
CECe: Effective Cationic Exchangeable Capacity
It is noteworthy that the pH values were in the acceptable range for most crops (between 5.6 and 8.4). In this context, the evaluation of soil pH is usually crucial, since it is associated with the availability of nutrients for plants and aluminum toxicity in the soil solution 19. In other soil characterization studies, it has been suggested that acid soils have medium to low exchangeable base contents, low usable P, medium to high Al saturation and medium CEC, and generally have limited solubility of Ca, Mg and K (18); however, this behavior did not occur at "El Mamey" farm. It is possible that the high availability of Ca and Mg is due to the formation processes and factors that have affected this area. It is known that 2:1 clays, montmorillonite group, produce high cation exchange capacity, which is in agreement with the results of the chemical analysis made for this soil, showing a high content of exchangeable cations and, in addition, the percentage of saturation by bases is high 15,21,22.
Table 4 shows the internutrient ratios calculated for different horizons identified in the soil profile. The Ca/Mg ratio was normal at all depths; however, the Ca/K; (Ca+Mg)/K; K/Mg and Mg/K ratios were out of range. The K/CECe ratio was low, but the Mg/CECe and Ca/CECe ratios were high. This means that, although Ca and Mg uptake by plants in this soil should be adequate, these elements should be blocking K uptake and, in turn, the latter element should not be predominant in the soil solution.
Table 4 Internutrient associations of the four horizons identified in the profile
| Horizon/Depth (cm) | Ca/Mg | Ca/K | (Ca+Mg)/K | K/Mg | Mg/K | K/CECe | Mg/CECe | Ca/CECe |
|---|---|---|---|---|---|---|---|---|
| A1(v)/0-22 | 5.23 | 216.56 | 257.96 | 0.02 | 41.40 | 0.39 | 15.95 | 83.42 |
| A2(v)/22-36 | 3.77 | 284.69 | 360.20 | 0.01 | 75.51 | 0.28 | 20.85 | 78.60 |
| C1g/36-59 | 2.36 | 188.24 | 268.07 | 0.01 | 79.83 | 0.34 | 27.44 | 64.70 |
| C2g/59-112 | 2.16 | 476.92 | 679.44 | 0.00 | 220.51 | 0.14 | 31.17 | 67.42 |
CECe: Effective Cationic Exchangeable Capacity
This behavior indicates that the internutrient equilibrium law is not being complied with in the soil and that it is necessary to correct this imbalance if adequate plant nutrition is to be achieved. In addition, the predominance of Ca2+ and Mg 2+ cations in the exchange complex is evident, which is common in soils with montmorillonite clay 21,23.
Table 5 presents the concentration of microelements and exchangeable Al present in the soil profile. Mn and Zn concentrations were medium in the first horizon and low in the rest. Fe was low in the whole horizon and Cu low in the first depth and medium in the rest. The exchangeable Al content was low from 0 to 36 cm depth, high from 36 to 59 cm and medium from 59 to 112 cm. Al saturation was low throughout the profile.
Table 5 Micronutrients and exchangeable aluminum concentration from the four identified horizons
| Horizon/Depth | Mn | Fe | Zn | Cu | Al | Al saturation |
|---|---|---|---|---|---|---|
| (cm) | (mg L-1) | (cmolc kg-1) | (%) | |||
| A1(v)/0-22 | 37.20 | 14.20 | 7.00 | 1.90 | 0.10 | 0.25 |
| A2(v)/22-36 | 7.70 | 7.80 | 1.70 | 2.10 | 0.10 | 0.28 |
| C1g/36-59 | 7.50 | 11.40 | 1.20 | 3.20 | 2.60 | 7.51 |
| C2g/59-112 | 6.70 | 13.40 | 1.00 | 2.70 | 0.70 | 1.27 |
It is important to point out that the natural concentrations of microelements in the soil depend fundamentally on the source material, the formation processes and the composition and proportion of the components in the solid phase. Other factors are the percentage and type of clay, organic matter content and physicochemical properties 24.
Coinciding with these authors, it has been suggested that in soils with high cation exchange capacity and with the presence of clays of the montmorillonite group, the amount of Fe, Zn and Cu microelements that may be present in soil is greater 14.
In addition, pH has been one of the most studied soil properties in relation to the availability of microelements, and it has been found that concentrations increase as pH becomes more acidic 23,25. Another factor that could have influenced in finding these levels of microelements was the burning of the soil before taking the soil samples, since it has been demonstrated that fire increases the availability of micronutrients in soil 20.
In spite of the above, it can be considered that the concentrations of microelements and Al found in the soil do not represent phytotoxicity levels for crops to be established; nevertheless, it is necessary to keep monitoring them, taking care that their concentrations do not increase to levels above those permissible at an international level.
Microbiological properties
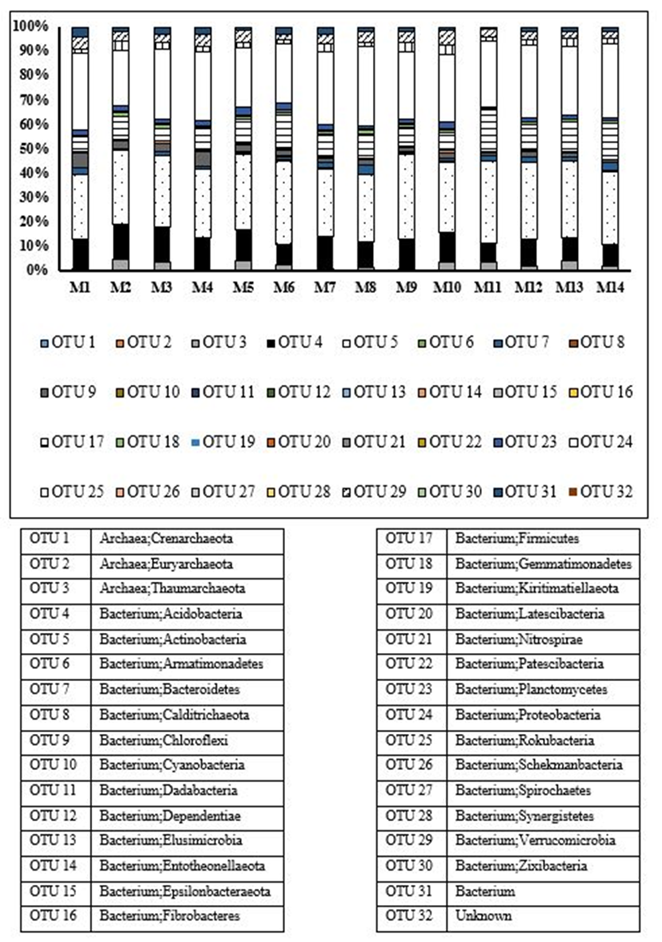
Figure 1 shows the relative abundance of phyla of Bacteria and Archaea found in the soil of the "El Mamey" farm. The most abundant were the phyla Actinobacteria, Proteobacteria, Firmicutes and Acidobacteria, all from the Bacteria domain. The other phyla found in the Bacteria and Archaea domains presented a lower relative abundance.
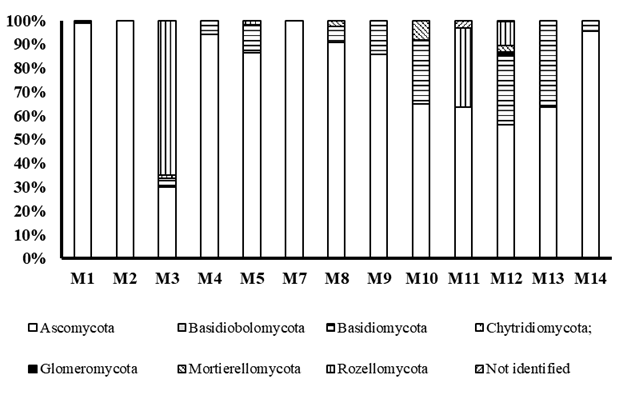
Figure 2 shows the relative abundance of fungal phyla found in the first 20 cm of the soil of the farm under study. In all samples, the predominance of the phylum Ascomycota was observed, followed by Basidiomycota and Rozellomycota.
Although metagenomic studies reveal the enormous diversity of microorganisms in the soil and the heterogeneity of their distribution, most of the sequences found belong to a few dominant phyla, and a large number of phyla were found with very low numbers of sequences, whether from the Bacterial and Archaeal domains or the Fungal Kingdom. It is also noteworthy the great diversity of phyla of Bacteria compared to that of fungi and the low relative abundance of the phylum Glomeromycota.
In particular, the primers used to study bacteria and archaea were designed and used satisfactorily for work carried out with samples from different sources, such as feces, soil, water, marine sediment, among others, in which the presence of a high diversity was found, as well as of orders and genera of the groups investigated 13,26.
On the other hand, the analysis of fungal populations present in soils is more complicated. Several studies have reported differences in the results regarding the proportion of the different fungal groups, at the phylum, order, genus and species level, depending on the use of different primer combinations and even the region sequenced 27-30.
In fact, it has been pointed out that the primer used here for its study ITS4A preferentially amplifies ascomycetes 31,32, which is in agreement with results, being the most abundant group. Another example is the study carried out in olive tree (Olea europeae L.) farms with the use of primers that amplify Glomeromycota fungi, where, contradictorily, 89.8 % of sequences belonging to organisms of a non-fungal nature were reported 33.
Regarding the variations of microorganism populations among samples, these depend directly on the availability of nutrients in the soil, which they use as substrate, and this availability is not usually uniform in space and time. Plant diversity increases the taxonomic and functional diversity of the soil microorganism community. This is because different plants generate different organic residues, resulting in a diversified food base 34. In this case, although the soil was previously covered with a grass species, this was not the only plant present on the site prior to the fire and subsequent land preparation.
In relation to this management type, fire before land preparation, other authors have already pointed out that fire has a direct and indirect influence, in the short and long term, on the biomass of soil microorganisms and their ecosystem services. The direct effect is due to the fact that high temperatures cause mortality and the indirect effects are related to changes in soil properties: substrate quality, nutrient concentration and soil humidity. Regarding fungal populations, fire stimulates phylum fungi populations of the Ascomycota and decreases the richness of AMF species by up to 14 % 20.
It is necessary to point out that relative abundance is a category of species diversity and intends to include species richness and the uniformity of their distribution in a simple expression and that the greater abundance of morphotypes and genera corresponds to studies carried out in natural ecosystems, which indicates greater diversity of microorganisms in comparison with agroecosystems 34,35.
In this regard, the microbial communities of protected forests are different from the patches of forest adjacent to productive fields, and these microbiomes are modified over the years of agricultural use. This modification does not occur at the loss diversity level , but through the modification of the relative abundances of several microbial groups, especially the less numerous ones 3.
However, despite the amount of information generated by massive DNA sequencing methodologies, to understand the functioning and dynamics of microbial systems, the new techniques must be complemented with other more traditional physicochemical determinations and with microbiological techniques that serve to evaluate functional changes to understand the behavior of the microbial community in relation to the biotic and abiotic factors of its environment 4.
CONCLUSIONS
The results indicate that it is a conserved soil, in which main limitations for its agricultural use are the high humidity retention, the low organic matter content and the imbalance in almost all the internutrient ratios evaluated.
It is possible to proceed with agricultural work, as long as the necessary corrections are made to achieve adequate plant nutrition.
It is necessary to continue checking properties studied over time, since the maintenance of the physical, chemical and biological properties of a soil are relevant aspects to avoid its degradation and erosion, when it is transformed from an area under pasture to an area destined for agricultural crops.
ACKNOWLEDGEMENTS
It would like to thank the National Research System of Panama (SNI-SENACYT) for their support in obtaining the data on the composition of the microorganism communities presented.
REFERENCES
1. Serrano-Montero DO, González-Paneque OS, de la Rosa-Andino AA, Aguilera-Corrales Y, Ramírez-Chávez RE. Estrategia de manejo y conservación del suelo en áreas de producción agrícola. Revista Ingeniería Agrícola [Internet]. 2017;7(1):41-8. Available from: https://www.rcta.unah.edu.cu/index.php/IAgric/article/download/512/5131. [ Links ]
2. John-Louis CM, Vantour-Causse A, Tamayo-Sierra AA. Estado de la fertilidad química de los suelos ferralíticos rojos de la granja Los Pinos. Revista Ingeniería Agrícola [Internet]. 2017;7(3):17-22. Available from: https://www.rcta.unah.edu.cu/index.php/IAgric/article/download/767/7692. [ Links ]
3. Soria MA. ¿Por qué son importantes los microorganismos del suelo para la agricultura? Química Viva [Internet]. 2016;15(2):3-10. Available from: https://www.redalyc.org/pdf/863/86347590002.pdf3. [ Links ]
4. Vera Macías L, Jiménez A, Gallo F, Guzmán Cedeño A, Cedeño A. Manual para la cartografía de suelos y la descripción de perfiles de suelos [Internet]. 1st ed. Escuela Superior Politécnica Agropecuaria de Manabí. Calceta, Manabí. Ecuador: Editorial Humus; 2017. 76 p. Available from: https://www.researchgate.net/publication/330968626_MANUAL_PARA_LA_CARTOGRAFIA_DE_SUELOS_Y_LA_DESCRIPCION_DE_PERFILES_DE_SUELOS_Adaptado_a_las_caracteristicas_de_los_suelos_de_la_parte_centro_norte_de_Manabi4. [ Links ]
5. Color (Firm) M. Munsell soil color charts [Internet]. rev. ed. New York: Macbeth, Division of Kollmorgen Instruments Corp., Munsell Color; 1992. 1 v. (loose-leaf): Available from: https://openlibrary.org/works/OL219096W/Munsell_soil_color_charts5. [ Links ]
6. Hernández-Jiménez A, Pérez-Jiménez JM, Bosch-Infante D, Speck NC. Clasificación Genética de Los Suelos de Cuba. Nueva Versión. 1st edicion. EDICIONES INCA; 2015. 93 p. [ Links ]
7. WRB IWG. World reference base for soil resources 2014 International soil classification system for naming soils and creating legends for soil maps. FAO [Internet]. 2015; Available from: https://publications.jrc.ec.europa.eu/repository/handle/JRC919477. [ Links ]
8. USDA, Departamento de Agricultura de los Estados Unidos Servicio de Conservación de Recursos Naturales. Claves para la taxonomía de suelos [Internet]. 2014 [cited 24/08/2021]. 410 p. Available from: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051546.pdf8. [ Links ]
9. Hernández JL. Métodos para el análisis físico de los suelos. Manual de laboratorio. Instituto Nacional de Ciencias Agrícolas (INCA). Ediciones INCA: La Habana, Cuba [Internet]. 2007; Available from: https://repositorioslatinoamericanos.uchile.cl/handle/2250/29142549. [ Links ]
10. Mesías-Gallo FW, Hernández-Jiménez A, Vera-Macías LR, Guzmán-Cedeño ÁM, Cedeño-Sacón ÁF, Ormaza-Cedeño KP, et al. Reservas de carbono orgánico en suelos de la llanura fluvial Calceta-Tosagua, Manabí, Ecuador. Cultivos Tropicales [Internet]. 2018 [cited 24/08/2021];39(4):27-33. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362018000400004&lng=es&nrm=iso&tlng=pt10. [ Links ]
11. Villarreal-Núñez JE, Name-Tuñón B, García-Espino RA. Monitoreo de cambios en la fertilidad de suelos por medio de análisis de laboratorio. Agronomía Mesoamericana [Internet]. 2012 [cited 24/08/2021];23(2):301-9. Available from: http://www.scielo.sa.cr/scielo.php?script=sci_abstract&pid=S1659-13212012000200009&lng=en&nrm=iso&tlng=es11. [ Links ]
12. Chávez AR. Comparación de dos métodos de determinación de la capacidad de intercambio catiónico en suelos de la región central de Honduras. Zamorano: Escuela Agrícola Panamericana, 2015. [Internet]. Available from: https://bdigital.zamorano.edu/handle/11036/456412. [ Links ]
13. Caporaso J, Lauber C, Walters W, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platformsOpen. The ISME Journal. 2012;6:1621-4. doi:10.1038/ismej.2012.8 [ Links ]
14. Yáñez Díaz MI, Cantú Silva I, González Rodríguez H, Yáñez Díaz MI, Cantú Silva I, González Rodríguez H. Efecto del cambio de uso de suelo en las propiedades químicas de un vertisol. Terra Latinoamericana [Internet]. 2018 [cited 24/08/2021];36(4):369-79. doi:10.28940/terra.v36i4.349 [ Links ]
15. Socarrás Armenteros Y, Hernández Jiménez A, Terry Alfonso E, González Cañizares PJ, Sánchez Iznaga ÁL, Delgado Cabrera O, et al. Cambios en las propiedades morfológicas de suelos pardos sialíticos sometidos a diferentes manejos agrícolas en Cuba. Idesia (Arica) [Internet]. 2019 [cited 24/08/2021];37(3):47-53. doi:10.4067/S0718-34292019000300047 [ Links ]
16. Hernández-Jiménez A, Pérez-Jiménez JM, Bosch-Infante D, Speck NC. La clasificación de suelos de Cuba: énfasis en la versión de 2015. Cultivos Tropicales [Internet]. 2019;40(1). Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362019000100015&script=sci_arttext&tlng=pt16. [ Links ]
17. Bryk M, Kołodziej B, Słowińska-Jurkiewicz A, Jaroszuk-Sierocińska M. Evaluation of soil structure and physical properties influenced by weather conditions during autumn-winter-spring season. Soil and Tillage Research [Internet]. 2017;170:66-76. Available from: https://www.sciencedirect.com/science/article/pii/S016719871730054517. [ Links ]
18. Calderón-Medina CL, Bautista-Mantilla GP, Rojas-González S. Propiedades químicas, físicas y biológicas del suelo, indicadores del estado de diferentes ecosistemas en una terraza alta del departamento del Meta. Orinoquia [Internet]. 2018;22(2):141-57. Available from: https://www.redalyc.org/jatsRepo/896/89660465002/89660465002.pdf18. [ Links ]
19. Guzmán G, Cabezas JM, Sánchez-Cuesta R, Lora Á, Bauer T, Strauss P, et al. A field evaluation of the impact of temporary cover crops on soil properties and vegetation communities in southern Spain vineyards. Agriculture, Ecosystems & Environment [Internet]. 2019 [cited 24/08/2021];272:135-45. doi:10.1016/j.agee.2018.11.010 [ Links ]
20. Singh AK, Kushwaha M, Rai A, Singh N. Changes in soil microbial response across year following a wildfire in tropical dry forest. For. Ecol. Manag. 2017;391: 458-468 [Internet]. Available from: https://www.sciencedirect.com/science/article/abs/pii/S037811271630639920. [ Links ]
21. Bossi J, Celio A, Mármol S. Formación Libertad: su reformulación. Agrociencia (Uruguay) [Internet]. 2016 [cited 24/08/2021];20(1):36-44. Available from: http://www.scielo.edu.uy/scielo.php?script=sci_abstract&pid=S2301-15482016000100006&lng=es&nrm=iso&tlng=pt21. [ Links ]
22. Solís FAM, Tejeira R. Determinación mineralógica de la fracción arcilla en suelos de importancia agrícolas de la República de Panamá. Investigaciones agropecuarias [Internet]. 2019 [cited 24/08/2021];2(1):34-48. Available from: https://www.revistas.up.ac.pa/index.php/investigaciones_agropecuarias/article/view/106422. [ Links ]
23. del Carmen Blanco M, Letisia Díaz S, Mabel Amiotti N. Geodisponibilidad de Co, Cr, Fe, Mo, Ni y Zn en la cuenca A° el divisorio. Ciencia del suelo [Internet]. 2017;35(1). Available from: http://www.suelos.org.ar/publicaciones/volumen3512017/161-170%20p%C3%A1gs%20CS%20433%20Blanco%20imprenta%20agost%2011.pdf23. [ Links ]
24. Martínez Robaina A, González JM, Sobrinho N, Odio M. Concentraciones y disponibilidad de metales pesados en suelos destinados al cultivo del tabaco localizados en la llanura sur de Pinar del Río-Cuba. 2020. doi:10.29327/ivsimposioabc.238141 [ Links ]
25. Huang J, Yuan F, Zeng G, Li X, Gu Y, Shi L, et al. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere [Internet]. 2017 [cited 24/08/2021];173:199-206. doi:10.1016/j.chemosphere.2016.12.137 [ Links ]
26. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences [Internet]. 2011 [cited 24/08/2021];108(Supplement 1):4516-22. doi:10.1073/pnas.1000080107 [ Links ]
27. Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environmental Microbiology [Internet]. 2010 [cited 24/08/2021];12(8):2165-79. doi:10.1111/j.1462-2920.2009.02099.x [ Links ]
28. Kohout P, Sudová R, Janoušková M, Čtvrtlíková M, Hejda M, Pánková H, et al. Comparison of commonly used primer sets for evaluating arbuscular mycorrhizal fungal communities: Is there a universal solution? Soil Biology and Biochemistry [Internet]. 2014 [cited 24/08/2021];68:482-93. doi:10.1016/j.soilbio.2013.08.027 [ Links ]
29. Van Geel M, Busschaert P, Honnay O, Lievens B. Evaluation of six primer pairs targeting the nuclear rRNA operon for characterization of arbuscular mycorrhizal fungal (AMF) communities using 454 pyrosequencing. Journal of Microbiological Methods [Internet]. 2014 [cited 24/08/2021];106:93-100. doi:10.1016/j.mimet.2014.08.006 [ Links ]
30. Berruti A, Desirò A, Visentin S, Zecca O, Bonfante P. ITS fungal barcoding primers versus 18S AMF-specific primers reveal similar AMF-based diversity patterns in roots and soils of three mountain vineyards. Environmental Microbiology Reports [Internet]. 2017 [cited 24/08/2021];9(5):658-67. doi:10.1111/1758-2229.12574 [ Links ]
31. Larena I, Salazar O, González V, Julián MC, Rubio V. Design of a primer for ribosomal DNA internal transcribed spacer with enhanced specificity for ascomycetes. Journal of Biotechnology [Internet]. 1999 [cited 24/08/2021];75(2):187-94. doi:10.1016/S0168-1656(99)00154-6 [ Links ]
32. Nikolcheva LG, Bärlocher F. Taxon-specific fungal primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycological Progress [Internet]. 2004 [cited 24/08/2021];3(1):41-9. doi:10.1007/s11557-006-0075-y [ Links ]
33. Montes-Borrego M, Metsis M, Landa BB. Arbuscular Mycorhizal Fungi Associated with the Olive Crop across the Andalusian Landscape: Factors Driving Community Differentiation. PLOS ONE [Internet]. 2014 [cited 24/08/2021];9(5):e96397. doi:10.1371/journal.pone.0096397 [ Links ]
34. Dias L a. F, Lopes I de ON, Cattelan AJ, Debiasi H, Sibaldelli RNR, Kosinsk CL, et al. Sistemas de cultivo utilizados na cultura da soja e efeito sobre a comunidade microbiana do solo. 2018 [cited 24/08/2021]; Available from: http://www.infoteca.cnptia.embrapa.br/handle/doc/110065134. [ Links ]
35. Furrazola E, Torres-Arias Y, Hernández-Prado R, Coronill YG. Hongos micorrizógenos arbusculares (Glomeromycota) en suelos agrícolas de la provincia Artemisa, Cuba. Acta Botánica Cubana [Internet]. 2019;218(1):34-43. Available from: https://www.researchgate.net/profile/Eduardo-Furrazola-Gomez-2/publication/333677432_Hongos_micorrizogenos_arbusculares_Glomeromycota_en_suelos_agricolas_de_la_provincia_Artemisa_Cuba_Arbuscular_mycorrhizal_fungi_Glomeromycota_in_agricultural_soils_of_Artemisa_province_Cuba/links/5cfea432a6fdccd13091be47/Hongos-micorrizogenos-arbusculares-Glomeromycota-en-suelos-agricolas-de-la-provincia-Artemisa-Cuba-Arbuscular-mycorrhizal-fungi-Glomeromycota-in-agricultural-soils-of-Artemisa-province-Cuba.pdf35. [ Links ]
Received: September 15, 2020; Accepted: June 26, 2021