Cultivos Tropicales
ISSN 1819-4087
30--2021
Original article
Growth and source-sink relationship in carrot plants biostimulated with Quitomax® and Pectimorf®
1Departamento Agronomía. Universidad de Matanzas, carretera Varadero km 3½, Matanzas, Cuba
2Instituto de Investigaciones de Ingeniería Agrícola (IAgric), carretera de Fontanar km 2½, Reparto Abel Santamaría, Boyeros, La Habana, Cuba
3Instituto Nacional de Ciencias Agrícolas (INCA), carretera San José-Tapaste, km 3½, Gaveta Postal 1, San José de las Lajas, Mayabeque, Cuba. CP 32 700
The research was developed with the aim of evaluating the influence of chitosan oligosaccharins and oligogalacturonides on the growth and source-sink relationship of the carrot crop. A randomized block experimental design with three replicates was established to evaluate the foliar application of 150 mg ha-1 of QuitoMax® and Pectimorf® on plants at 20 and 50 days after planting (DAS). Periodic destructive sampling was carried out to determine, among other agromorphological variables: height, leaf area, accumulation and distribution of dry mass in the plant, from which growth rates and source-sink potentials were calculated throughout the biological cycle. At harvest, crop length, diameter, fleshy root weight and yield were evaluated. A direct effect of both oligosaccharins on photosynthetic activity, production and distribution of photoassimilates from leaves to roots in early stages of the biological cycle was evidenced. The results validated the favorable effect of the biostimulants on plant growth and dry mass production with a significant impact of the PectiMorf® product (oligogalacturonide mixture) on the commercial quality of the fleshy root and yield at harvest.
Key words: oligosaccharides; plant physiology; biomass production; yield
INTRODUCTION
Carrot (Daucus carota L.) is the most widespread commercial species of the Umbelliferae family and one of the most widely grown root vegetable crops worldwide, with high economic values 1. In the last 30 years, the growth rate of its consumption has exceeded the population growth rate on a global scale. China is the main producer country with about 16 800 000 tons, followed by Russia (1 565 032 t), the United States (1 346 080 t), Uzbekistan (1 300 000 t) and Ukraine (915 900 t), most of which is marketed in fresh form 2. The world average yield is 22.4 t ha-1, with countries such as Holland, Spain, England and USA standing out with values between 40 and 50 t ha-1 (3.
In Cuba, yields reach 30 to 40 t ha-1, depending on the variety and production conditions; it is one of the most sinked vegetables at any time of the year, due to its excellent taste qualities, the possibility of being consumed fresh or canned, and its relative contribution in vitamins and minerals 4.
Its crop in the country extends, fundamentally, to organoponic conditions and intensive orchards under sustainable production technologies (minimum use of agrochemicals), dependent on high volumes of organic matter not always available, which limits the fertility of the substrates and the biological efficiency of plants. For this reason, the use of biostimulants and biofertilizers is implemented to allow the crop to overcome stress situations in adverse environmental conditions and maximize its intrinsic productive potential to increase yields 5.
In this sense, the biostimulants QuitoMax® (a mixture of chitosan polymers) and PectiMorf® (a mixture of pectic oligosaccharides or oligogalacturonides), developed by the National Institute of Agricultural Sciences (INCA), have proven to be a promising alternative to positively induce the growth and productivity of several crops, since they exert a proven influence on vegetative and root growth, shorten and enhance the flowering and fruiting period and increase yields through various application forms 6-10.
The present work aims to evaluate QuitoMax® and Pectimorf® biostimulant effect on growth and the source-sink relationship of carrot crops.
MATERIALS AND METHODS
The research was carried out in the Organoponic of the University of Matanzas (23º 01ʹ 57ʺ N and -81º 30ʹ 31ʺ W, Lambert Conformal Conical Projection with origin Cuba North), under intensive orchard conditions, during February to May 2017. The carrot variety "New Kuroda" with a biological cycle of 90 to 100 days was used.
An area of 180 m2 was selected for the experiment, with typical red Ferrallitic soil 11. A randomized block design with three treatments and three replicates was established, consisting of 20 m2 plots (nine experimental plots). A planting frame of 0.10 m between plants and 0.15 m between rows (four rows per bed) was taken into account. The following treatments were evaluated:
T1: control, no product application.
T2: foliar application of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS.
T3: foliar application of 150 mg ha-1 of Pectimorf® at 20 and 50 DAS.
The doses were selected taking into account the favorable results obtained in other crops with the use of these biostimulants 12,13.
The QuitoMax® product was characterized by presenting chitosan polymers with an average molecular mass of 1.35 x 105 g mol-1, a degree of N-acetylation of 12 % and a concentration of 4 g L-1, while PectiMorf® presented a composition between 55 and 61 % of galacturonid acid and an active ingredient concentration of 0.75 g L-1.
The application was carried out with a 16 liter Matabi knapsack, wetting all plants homogeneously, at a rate of 0.1 µL plant-1 for QuitoMax® and 0.5 µL plant-1 for PectiMorf®.
Agrotechnical management was carried out taking into account the Technical Guide for the Production of Carrot Crops 4, modified according to the possibilities of the experimental area and the crop development, without applying fertilizers and phytosanitary products. The water needs of the crop were covered with the use of a microjet irrigation system.
The evaluations were carried out at different times of the crop cycle (30, 50, 70 and 90 DAS) by destructive sampling, determining each time, in 10 plants per plot (30 per treatment): height (cm), leaf area (dm2) from the number of active leaves with the use of ImageJ software ver. 1.51 software 14 and the proposed methodologies 15,16; total dry mass (g) and by organs (leaves and stem-root complex). Growth rates were calculated using the expressions shown in Table 1 17.
Table 1 Formulas and units for determining growth rates in vegetables
| Growth index | Symbols | Formulas | Units |
|---|---|---|---|
| Leaf Area Index | LAI |
|
Dimensionless |
| Net Assimilation Rate | NAR |
|
g dm-2 day-1 |
| Crop Growth Rate | CGR |
|
g m-2 day-1 |
Symbols Used: W=Total Dry Mass; T=Time; LA=Leaf Area; SA=Soil Area; NL= Natural Logarithm
From the values of dry mass per organ, the distribution curve of assimilates during the crop cycle and the strength or power of source and plant sink were determined 18. The data were analyzed in terms of plant:
Source power = Source size (Leaf area)*Source activity (NAR)
Sink power = Sink size (Root dry matter)*Sink activity (TRC Root)
At harvest, fleshy root length and diameter (cm), as well as fresh mass (g) were evaluated and crop agricultural yield (t ha-1) was estimated.
Data were processed using Statgraphics Plus v.5.1 19, using a simple rank ANOVA and means were compared by Duncan's Multiple Range test at a p≤0.05; in the analysis of dynamics, the standard error of the means was estimated.
RESULTS AND DISCUSSION
Plant height did not show significant differences between treatments during the phenological crop development (Table 2). However, an increase in this variable was observed in plants treated with biostimulants with respect to the control, starting at 50 DAS.
Table 2 Carrot plant height (cm) in the samplings carried out
| Treatments* | Height plant (cm) | |||
|---|---|---|---|---|
| 30 DAS | 50 DAS | 70 DAS | 90 DAS | |
| T1 | 23,5 ± 2,66 | 27,86 ± 2,68 | 38,89 ± 7,06 | 46,83 ± 5,36 |
| T2 | 24,27 ± 1,54 | 29,91 ± 3,71 | 42,06 ± 7,45 | 51,22 ± 5,09 |
| T3 | 25,33 ± 2,82 | 29,2 ± 4,97 | 45,06 ± 8,38 | 50,0 ± 8,02 |
* T1: control without application, T2: foliar spraying of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spraying of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS ± Standard Error
QuitoMax® and PectiMorf® stimulating effect on plant growth has been validated by many authors 20-24. This effect is attributed to the fact that oligosaccharins function as hormonal chemical messengers that regulate growth mechanisms and differentiation in different crops, accelerating the growth process of plants 25. In addition, it is referred that they can stimulate photosynthetic activity; therefore, there is a greater gain of carbon skeletons that are used for the synthesis of new compounds, such as proteins 26.
Figure 1 shows the efficiency of bioproducts in the development of crop leaf area. The best results were achieved in treated plants as a consequence of an increase in leaf size, the oligogalacturonide mixture caused the greatest increase with 25 % above the control, but with no difference with chitosan, the latter did not differ statistically from the control.
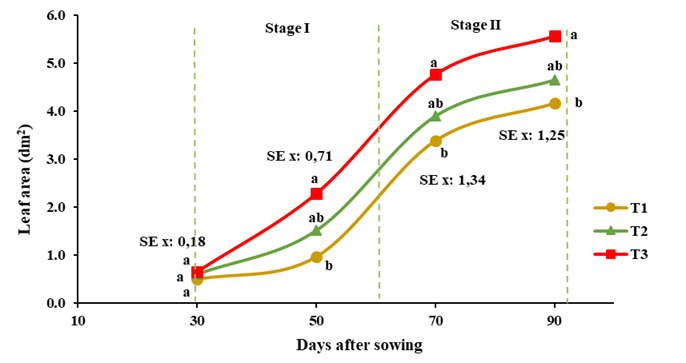 T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters in the vertical indicate significant differences according to the Duncan test for p≤0.05 in the analysis of each sample
T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters in the vertical indicate significant differences according to the Duncan test for p≤0.05 in the analysis of each sampleFigure 1 Dynamics of leaf area (dm2) of the carrot plant during the growing cycle.
It is characteristic in carrot plants to observe a slow growth of foliage in the first stage of vegetative development (30-60 DAS), which then increases steadily in number of leaves between 61 and 97 DAS (Stage II); then the growth of foliage slows down (reproductive development phase) and towards the end of the biological cycle of the crop the foliar area tends to stabilize 27. However, the application of biostimulants achieved a higher increase in plant leaf area growth in the first stage, compared to the second, of 26 and 13 % for the oligogalacturonide mixture and chitosan, respectively.
The foliar development seen with Pectimorf® application can be attributed to the fact that this product was able to stimulate the appropriate endogenous hormonal balance, to activate the increase of cell division in buds that originate leaves and promote the synthesis of important substances that act in these processes. Although the mechanisms that can explain in depth the influence of oligogalacturonides on cell division in higher plants have not been defined 28, it has been observed that they are capable of triggering a series of stimuli that accelerate metabolism and enzymatic activity in the cells, which enhances plant growth and development 29.
It can also be related to the increase of photosynthetic efficiency of plant, since it has been observed that the application of this biostimulant increases the content of photosynthetic pigments (chlorophyll a, b and total) in the leaves 26,30,31, which allows a greater capture of photosynthetically active radiation and, therefore, an increase in photosynthetic activity. Other studies indicate that Pectimorf® can enhance photosynthetic capacity in plants due to its effect on the modification of stomatal development patterns 25.
It is reported that the incorporation of oligogalacturonide mixture in in vitro culture media and its foliar spraying in the acclimatization phase increased the number of leaves in banana seedlings (Musa spp., AAAB) cultivars FHIA 18 8) and FHIA 21 32, as well as the leaf area in seedlings of papaya (Carica papaya L.) cultivar Maradol Roja 33) and pineapple (Ananas comosus var. comosus) hybrid MD-2 34.
The second application of products accelerated the rate of foliage growth, which had a marked influence on the production of assimilates in plants. The increase in plant biomass is realized from the expanded leaf area as a result of a greater net photosynthetic activity, which is a critical variable for productivity 34.
The increase in dry mass was similar at 30 DAS in all treatments (Figure 2). Statistically significant differences were found after 50 DAS; at the end of the crop cycle, the treatments with both biostimulants showed the highest productivity (11.76 and 12.58 g plant-1, respectively) with increases of 45 and 55 % with respect to the control (8.1 g plant-1), which at all times evaluated presented the lowest absolute values.
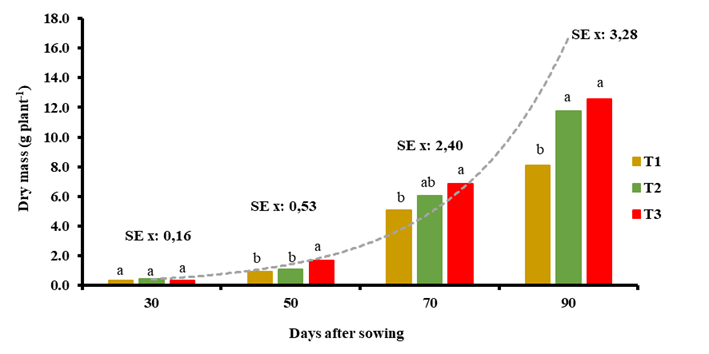 T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters indicate significant differences according to Duncan test for p≤0.05 in the analysis of each sampling
T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters indicate significant differences according to Duncan test for p≤0.05 in the analysis of each samplingFigure 2 Total dry mass accumulation (leaves and stem-root complex) (g) in carrot plants at different times of the crop cycle
Oligosaccharins favor plant photosynthetic activity, causing greater biomass accumulation in both aerial organs and roots. Previous authors have shown that foliar PectiMorf® application causes increases in the aerial and root mass of radish 35, as well as greater productivity of accumulated biomass in potato tubers 36) and zucchini fruits (Cucurbita pepo L.) var. 'Grey Zucchini' 37. Also, different forms of treatment with QuitoMax® have increased the formation and enlargement of stolons in potato 22, the production of fresh and dry mass in cucumber fruits 38 and peppers 39, as well as the aerial part and roots in tobacco plants 24.
The determination of growth indices (Figure 3) allowed a better understanding of the growth process and physiological efficiency of the carrot crop. No significant differences were found in the LAI and NAR indicators in plants treated with the biostimulators, although they did show a better performance with respect to the non-applied control.
 T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters in the vertical indicate significant differences according to the Duncan test for p≤0.05 in the analysis of each sample
T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters in the vertical indicate significant differences according to the Duncan test for p≤0.05 in the analysis of each sampleFigure 3 Carrot crop growth indices: Leaf Area Index (A), Net Assimilation Rate (B), Crop Growth Rate (C)
Crop growth rate increased as the crop cycle progressed and the formation of the fleshy root began; it was the only one of the indices studied that showed statistically significant differences. The highest values coincided with the moment when there was an increase in the sink potential (70 DAS for the control and between 70-90 DAS with biostimulant application). PectiMorf® application caused the highest values, followed by the QuitoMax® treatment, which in the first sampling did not differ from the control. At the end of the crop cycle, the biostimulated plants recorded a maximum value of 19.0 g m-2 day-1, while the untreated plants only reached increases of 10.85 g m-2 day-1 which shows an increase of 75 % with the use of the products.
The results support the hypothesis that biostimulants favor plant photosynthetic activity 40, although it cannot be ruled out that, in addition, these products facilitate the uptake of nutrients from the soil, something that has been previously demonstrated 41. Thus, Pectimorf®, due to its potential as a rooting agent 33-42, can favor the formation of roots that enable an efficient supply of water and mineral salts 34 and, therefore, greater plant development.
Differences were also found in the treatments evaluated in terms of the maximum points of dry mass accumulation in the plant organs (Figure 4).
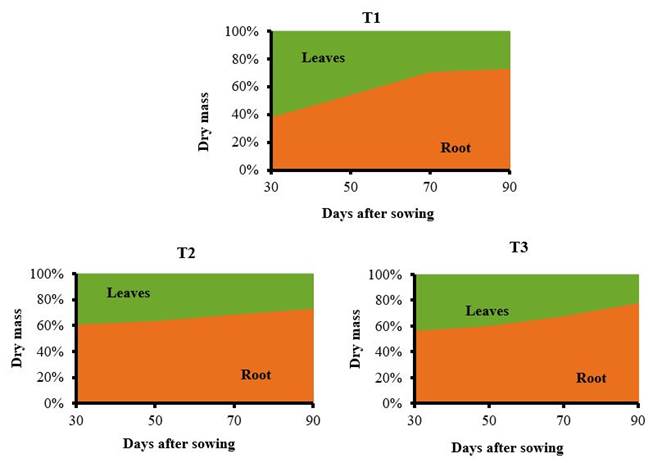 T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS
T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DASFigure 4 Dynamics of dry mass percentage distribution by organs (leaves and root) with respect to the total in carrot plants
The control treatment showed normal behavior in the distribution of dry biomass. A higher percentage of accumulation was observed in the leaves, at 30 DAS, with 61.4 % of the total. Dry mass accumulation in the root was a slow process up to that time, but then began to increase steadily until harvest, where 73.7 % was reached.
To achieve rapid initial growth of young carrot plants, a substantial increase in leaf area in the vegetative phase is important because, due to the morphology of leaves, which have long petioles and segmented blades, much of the incident solar radiation is not intercepted. Therefore, in this phase, a large part of assimilates must be destined to the formation of leaves 43. When the foliar system reaches an appropriate development, the partitioning of assimilates to the foliage decreases, with the consequent increase in their mobilization to the root 44. With the onset of thickening, roots become the main sink.
Plants treated with biostimulants showed a greater accumulation of assimilates in the root (61.8 and 56.9 % for QuitoMax® and PectiMorf® applications, respectively) than in the leaves (38.2 and 43.1 %) from the first sampling.
The partitioning of assimilates between the aerial part and the root, during the carrot crop cycle, is an expression of the interaction between genetic, environmental and cultural factors 45. The observed behavior can be attributed to the fact that the foliar application of bioproducts increased levels of traditional hormones such as gibberellins and abscisic acid (ABA), substances that are closely related to the process of dry mass distribution in plants and the tuberization induction 46.
Figure 5 shows differences in the behavior of the source-sink potentials of plants in the evaluated treatments. In the control, the maximum potential of leaves as source tissue was obtained at 70 DAS, with a value of 0.35 g day-1; the same behavior was presented at this time by the root sink potential with a value of 0.38 g day-1. This period corresponded to the moment of greatest translocation of assimilates from leaves to roots. At the end of the cycle for this treatment, both potentials decreased as a result of the decrease in the LAI and the rate of root weight gain near harvest.
Plants that received QuitoMax® applications reached values above 70 DAS, with maximum source and sink potentials for leaves and roots of 0.46 and 0.40 g day-1, respectively; towards the end of the crop development cycle the source potency decreased markedly, while the sink strength maintained high values (0.34 g day-1) indicating that the root continued to accumulate reserves until harvest time, increasing crop productivity.
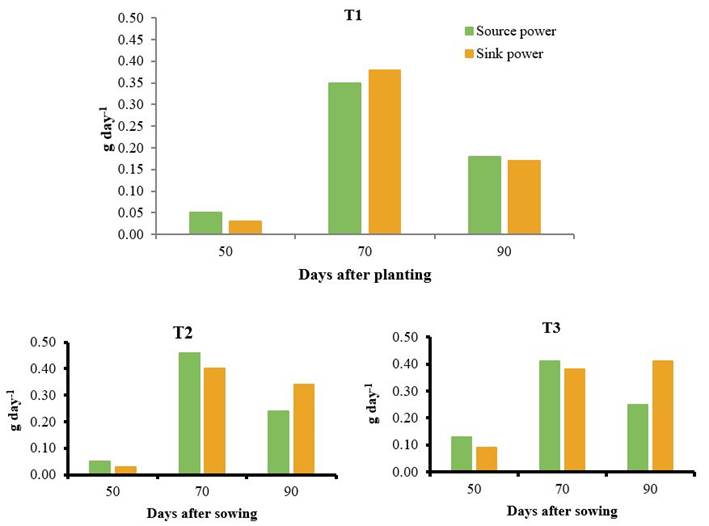 T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS, T4: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS
T1: control without application, T2: foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS, T4: foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DASFigure 5 Source-sink potentials in carrot plants
On the other hand, the application of PectiMorf® caused a higher source power in plants at 70 DAS (0.41 g day-1) and a higher root sink potential at 90 DAS (0.41 g day-1). In the latter sampling, a considerable increase in sink power was observed, which represents, that the highest translocation and discharge of photoassimilates in the root took place in this period.
Dry mass assimilation and its distribution within the plant are important processes that determine crop productivity 47. The production of assimilates by the leaves and the point to which they can be accumulated by the sink, represented in this case by the harvested root, defines yields 48. Higher values of the potentials between source and sink organs and in the net assimilation rate (NAR) can translate into a greater biomass accumulation at times close to physiological maturity, reaching higher yields 49.
Table 3 shows results on yield and the components that determine it at the time of harvest. The length of the fleshy root did not differ between treatments, but the diameter, the mass of the fleshy root and, therefore, the yield were higher in plants treated with PectiMorf® and differed significantly from the control and chitosan application.
Table 3 Yield and its components in carrot plants
| Treatments* | Fleshy root length (cm) | Fleshy root diameter (cm) | Fleshy root mass (g)) | Yield (t ha-1) |
|---|---|---|---|---|
| T1 | 10,14 ± 2,80 | 2,41 ± 0,36 b | 47,85 ± 8,05 b | 31,5 ± 2,10 b |
| T2 | 11,22 ± 2,53 | 2,56 ± 0,44 b | 58,60 ± 7,63 b | 38,70 ± 1,25 b |
| T3 | 11,39 ± 1,39 | 3,33 ± 0,25 a | 74,41 ± 17,46 a | 49,11 ± 3,26 a |
* T1: Control without application, T2: Foliar spray of 150 mg ha-1 of QuitoMax® at 20 and 50 DAS, T3: Foliar spray of 150 mg ha-1 of PectiMorf® at 20 and 50 DAS. Different letters in the vertical indicate significant differences according to the Duncan test for p≤0.05 in the analysis of each sample. ± Standard Error
Biostimulants had a positive effect on the agricultural yield of carrot, proving to be effective in the productivity of treated plants, taking into account the increases with respect to the control of 22 % with QuitoMax® (although without differences with the control) and 56 % with PectiMorf®. Results achieved are superior to those reported for this same variety with the use of biofertilizers based on efficient microorganisms 50.
In the literature, there are no antecedents of the use of oligosaccharins in carrot cultivation, but it has been demonstrated that applications of PectiMorf® increase yields in radish 35, garlic 7 and potato 9,21,36, as well as the quality of the tubers obtained. The positive effect of the oligogalacturonide mixture on the yield of other non-tuberous crop species such as beans 13, pumpkin 37 and papaya 51 has also been corroborated.
Oligosaccharins (and products containing them) have been reported as important yield biostimulatory macromolecules in several crops, with increases ranging from 10 to 60 % above controls, depending on the application rates experimented, the crop and the locations and soils treated 52-54.
CONCLUSIONS
The carrot crop responded favorably to the foliar application of biostimulants, with a significant impact of the product PectiMorf® (T3) on the increase in leaf area and dry mass production. The differences found in the crop growth rate (CGR), the higher increases in the net assimilation rate (NAR) and the differences in the behavior of the source-sink potentials of plants, with the application of the biostimulants, show their direct effect on the photosynthetic activity, the production and distribution of photoassimilates from leaves to roots in early stages of the biological cycle.
The foliar application of 150 mg ha-1 of PectiMorf® at 30 and 50 days after planting showed a better productive response of the plants and the commercial quality of the fleshy root, expressed in a notable increase in yields.
BIBLIOGRAFÍA
1. Nan H, Gao LZ. Genome-wide analysis of WRKY genes and their response to hormone and mechanic stresses in carrot. Front Genet [Internet]. 2019;10:363. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2019.00363/full1. [ Links ]
2. Ávila C. Manual Zanahoria [Internet]. Bogotá, Colombia; 2015 p. 50. (Programa de apoyo agrícola y agroindustrial vicepresidencia de fortalecimiento empresarial). Available from: https://www.dane.gov.co/files/investigaciones/agropecuario/sipsa/Bol_Insumos_jun_2017.pdf2. . [ Links ]
3. Gaviola JC. Manual de producción de zanahoria. Mendoza, Argentina: INTA [Internet]. 2013;97-8. Available from: https://inta.gob.ar/sites/default/files/script-tmp-inta_-_prlogo_e_ndice.pdf3. [ Links ]
4. Pérez Rodríguez PJ, Figueredo Sánchez M. Guía técnica para la producción del cultivo de la zanahoria [Internet]. Primera Edición. Bejucal-Quivicán, La Habana. Cuba: Instituto de Investigaciones Hortícolas "Liliana Dimitrova; 2009 [cited 28/08/2021]. 13 p. Available from: https://docplayer.es/18791081-Guia-tecnica-para-la-produccion-del-cultivo-de-la-zanahoria.html4. [ Links ]
5. Núñez Sosa DB, Liriano González R, Pérez Hernández Y, Placeres Espinosa I, Sianeh Zawolo G. Respuesta de Daucus carota5. L. a la aplicación de microorganismos nativos en condiciones de organopónico. Centro Agrícola [Internet]. 2017;44(2):29-35. Available from: http://scielo.sld.cu/scielo.php?pid=S0253-57852017000200004&script=sci_arttext&tlng=pt5. [ Links ]
6. Falcón Rodríguez AB, Costales Mené D, González-Peña Fundora D, Nápoles García MC. Nuevos productos naturales para la agricultura: las oligosacarinas. Cultivos Tropicales [Internet]. 2015;36:111-29. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-593620150005000106. [ Links ]
7. Izquierdo H, Diosdado E, Cepero MCG, de la C Núñez M, Cabrera JC, Hernández RM, et al. Contributions to knowledge of the functioning of national bioestimulators in plant biotechnology processes. Biotecnología Aplicada [Internet]. 2016;33(3):3511-6. Available from: https://www.medigraphic.com/cgi-bin/new/resumenI.cgi?IDREVISTA=281&IDARTICULO=72516&IDPUBLICACION=70577. [ Links ]
8. Morales-Guevara D, Dell Amico-Rodríguez J, Jerez-Mompie E, Rodríguez-Hernández P, Álvarez-Bello I, Díaz-Hernández Y, et al. Efecto del QuitoMax(r) en plantas de frijol (Phaseolus vulgaris8. L.) sometidas a dos regímenes de riego. I. Crecimiento y rendimiento. Cultivos Tropicales [Internet]. 2017;38(2):119-28. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-593620170002000188. [ Links ]
9. Jerez-Mompie E, Morales-Guevara D, Dell Amico-Rodríguez J, Falcón-Rodríguez A. El Quitomax(r) influye en la producción de tubérculos semilla de papa (Solanum tuberosum9. L.) variedad Romano. Cultivos Tropicales [Internet]. 2018;39(3):80-6. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-593620180003000119. [ Links ]
10. Pandey P, Verma MK, De N. Chitosan in agricultural context-A review. Bull. Environ. Pharmacol. Life Sci [Internet]. 2018;7:87-96. Available from: https://www.researchgate.net/profile/Priyal-Pandey/publication/326682315_Chitosan_in_agricultural_context_-_A_review/links/5b72ddc792851ca6505d7c61/Chitosan-in-agricultural-context-A-review.pdf10. [ Links ]
11. Hernández JA, Pérez JJM, Bosch ID, Castro SN. Clasificación de los suelos de Cuba. Primera Edición. Mayabeque, Cuba: EDICIONES INCA; 2015. p. 91. Available from: http://ediciones.inca.edu.cu/files/libros/clasificacionsueloscuba_%202015.pdf11. [ Links ]
12. Morales Guevara D, Torres Hernández L, Jerez Mompié E, Falcón Rodríguez A, Amico Rodríguez JD. Efecto del Quitomax(r) en el crecimiento y rendimiento del cultivo de la papa (Solanum tuberosum12. L.). Cultivos Tropicales [Internet]. 2015;36(3):133-43. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362015000300020&script=sci_arttext&tlng=en12. [ Links ]
13. Dell Amico J, Morales D, Jerez E, Rodríguez P, Álvarez I, Martín R, et al. Efecto de dos variantes de riego y aplicaciones foliares de Pectimorf(r) en el desarrollo del frijol (Phaseolus vulgaris13. L.). Cultivos Tropicales [Internet]. 2017;38(3):129-34. Available from: http://scielo.sld.cu/pdf/ctr/v38n3/ctr18317.pdf13. [ Links ]
14. Rasband W. ImageJ [Internet]. Version 1.51j8. National Institutes of Health: USA. 2015. [cited 28/08/2021]. Available from: https://imagej.nih.gov/ij/download/14. [ Links ]
15. Por E, Sfair J. Usando o ImageJ para calcular a área foliar [Internet]. 2013. Available from: https://www.researchgate.net/publication/235737768_Usando_o_ImageJ_para_calcular_a_area_foliar15. [ Links ]
16. de la Caridad Aday Díaz O, González Hernández R, Díaz Mujica FR, Reyes Esquirol C, Gil Cruz Y, Reyes Pérez S, et al. Aplicación del software ImageJ(r) 1.43 u en la caracterización de los síntomas de la mancha anular de la caña de azúcar. Centro Agrícola [Internet]. 2017;44(2):83-8. Available from: http://scielo.sld.cu/scielo.php?pid=S0253-57852017000200011&script=sci_arttext&tlng=pt16. [ Links ]
17. Castellanos MS, Abril MS, López CEÑ. Análisis de Crecimiento y Relación Fuente-Demanda de Cuatro Variedades de Papa (Solanum tuberosum17. L.) en el Municipio de Zipaquirá (Cundinamarca, Colombia). Revista Facultad Nacional de Agronomía Medellín [Internet]. 2010;63(1):5253-66. Available from: https://revistas.unal.edu.co/index.php/refame/article/view/2494517. [ Links ]
18. Wilson JW. Analysis of growth, photosynthesis and light interception for single plants and stands. Annals of Botany [Internet]. 1981;48(4):507-12. Available from: https://academic.oup.com/aob/article-abstract/48/4/507/22323018. [ Links ]
19. Statistical Graphics Crop. STATGRAPHICS | Data Analysis Solutions [Internet]. 2000 [cited 28/08/2021]. Available from: https://www.statgraphics.com19. / [ Links ]
20. Costales D, Nápoles MC, Falcón AB, González Anta G, Ferreira A, Rossi A. Influencia de quitosanas en la nodulación y el crecimiento vegetativo de soya (Glycine max20. L. Merrill). Cultivos Tropicales [Internet]. 2017;38(1):138-46. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-5936201700010001820. [ Links ]
21. Jerez Mompie E, Martín Martín R, Morales Guevara D, Reynaldo Escobar I. Efecto de oligosacarinas en el comportamiento de la papa (Solanum tuberosum21. L.) variedad Romano. Cultivos Tropicales [Internet]. 2017;38(1):75-80. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362017000100008&script=sci_arttext&tlng=en21. [ Links ]
22. Rizo-Alvarez M, Morales-Querol D, Sánchez-Santana T, López-Vigoa O, Olivera-Castro Y, Benítez-Alvarez MA, et al. Influencia del EcoMic(r) y el Pectimorf(r) en el establecimiento de Leucaena leucocephala22. (Lam.) de Wit. cv. Cunningham. Pastos y Forrajes [Internet]. 2018;41(3):183-8. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-0394201800030000422. [ Links ]
23. Bécquer Granados CJ, González Cañizares PJ, Ávila Cordoví U, Nápoles Gómez JÁ, Galdo Rodríguez Y, Muir Rodríguez I, et al. Efecto de la inoculación de microorganismos benéficos y Quitomax(r) en Cenchrus ciliaris23. L., en condiciones de sequía agrícola. Pastos y Forrajes [Internet]. 2019;42(1):39-47. Available from: http://scielo.sld.cu/scielo.php?pid=S0864-03942019000100039&script=sci_arttext&tlng=en23. [ Links ]
24. González Gómez LG, Jiménez Arteaga MC, Paz Martínez I, Oliva Lahera A, Falcón Rodríguez A. Aplicación de QuitoMax(r) en semillas y posturas de tabaco en semillero. Centro Agrícola [Internet]. 2020;47(2):16-21. Available from: http://scielo.sld.cu/scielo.php?pid=S0253-57852020000200016&script=sci_arttext&tlng=en24. [ Links ]
25. Álvarez Bello I, Reynaldo Escobar IM. Efecto del Pectimorf(r) en el índice estomático de plantas de frijol (Phaseolus vulgaris25. L.). Cultivos Tropicales [Internet]. 2015;36(3):82-7. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362015000300013&script=sci_arttext&tlng=en25. [ Links ]
26. Izquierdo H, Núñez M, González MC, Proenza R, Cabrera JC. Influencia de un oligogalacturónido en la aclimatización de vitroplantas de banano (Musa spp.) del clon FHIA-18 (AAAB). Cultivos Tropicales [Internet]. 2009 [cited 28/08/2021];30(1):00-00. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362009000100005&lng=es&nrm=iso&tlng=es26. [ Links ]
27. Departamento Administrativo Nacional de Estadística (DANE). Características relevantes en el cultivo de la zanahoria (Daucus carota27. L.) en Colombia y estudios de caso sobre costos de producción en los municipios de Madrid (Cundinamarca) y Ventaquemada (Boyacá) [Internet]. 2017 p. 1-6. Available from: https://www.dane.gov.co/files/investigaciones/agropecuario/sipsa/Bol_Insumos_jun_2017.pdf27. [ Links ]
28. González-Pérez L, Vázquez-Glaría A, Perrotta L, Acosta A, Scriven SA, Herbert R, et al. Oligosaccharins and Pectimorf(r) stimulate root elongation and shorten the cell cycle in higher plants. Plant Growth Regulation [Internet]. 2012;68(2):211-21. Available from: https://link.springer.com/article/10.1007/s10725-012-9709-z28. [ Links ]
29. Enríquez-Guevara EA, Aispuro-Hernández E, Vargas-Arispuro I, Martínez-Téllez MÁ. Oligosacarinas derivadas de pared celular: Actividad biológica y participación en la respuesta de defensa de plantas. Revista mexicana de fitopatología [Internet]. 2010;28(2):144-55. Available from: http://www.scielo.org.mx/scielo.php?pid=S0185-33092010000200007&script=sci_abstract&tlng=pt29. [ Links ]
30. Ojeda Silvera CM. Efecto de un producto bioactivo compuesto por oligogalacturónidos como mitigador del estrés hídrico en variedades de albahaca (Ocimum basilicum30. L). [Internet] [Tesis Doctoral]. Centro de Investigaciones Biológicas del Noroeste; 2015. Available from: https://cibnor.repositorioinstitucional.mx/jspui/handle/1001/17030. [ Links ]
31. Izquierdo Oviedo H, Alcaraz Meléndez L, Rodríguez-Álvarez M. Micropropagación de chiltepín (Capsicum annuum31. L. cv.' glabriusculum') mediante el empleo de una oligosacarina de origen péctico. Acta universitaria [Internet]. 2017;27(5):34-43. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0188-6266201700050003431. [ Links ]
32. Mogena AF, García MB, Reisel DB. Uso del Pectimorf(r) en el enraizamiento in vitro de plantas del cultivar FHIA 21 (Musa spp., AAAB). Agricultura Tropical [Internet]. 2018;3(2). Available from: http://ojs.inivit.cu/index.php?journal=inivit&page=article&op=view&path%5B%5D=7532. [ Links ]
33. Posada-Pérez L, Padrón-Montesinos Y, González-Olmedo J, Rodríguez-Sánchez R, Barbón-Rodriguez R, Norman-Montenegro O, et al. Efecto del Pectimorf(r) en el enraizamiento y la aclimatización in vitro de brotes de papaya (Carica papaya L.) cultivar Maradol Roja. Cultivos Tropicales [Internet]. 2016;37(3):50-9. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362016000300005&script=sci_arttext&tlng=pt33. [ Links ]
34. Sánchez RR, Mbogholi A, Lorente GY, Rodríguez RC, Olmedo JLG. Efectos del ácido giberélico y el PectiMorf(r) en las vitroplantas de piña (Ananas comosus34. var. comosus34. ) 'MD-2' durante la fase final de aclimatización. Universidad&Ciencia [Internet]. 2019;8(1):135-47. Available from: https://core.ac.uk/download/pdf/287219496.pdf34. [ Links ]
35. Terry Alfonso E, Ruiz Padrón J, Tejeda Peraza T, Reynaldo Escobar I. Efectividad agrobiológica del producto bioactivo Pectimorf(r) en el cultivo del Rábano (Raphanus sativus35. L.). Cultivos Tropicales [Internet]. 2014;35(2):105-11. Available from: http://scielo.sld.cu/scielo.php?pid=S0258-59362014000200014&script=sci_arttext&tlng=en35. [ Links ]
36. Martín-Martín R, Jerez-Mompie E, Morales-Guevara D, Reynaldo-Escobar I. Empleo de Pectimorf(r) para estimular la tuberización en papa (Solanum tuberosum36. L.). Cultivos Tropicales [Internet]. 2017;38(3):72-6. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-5936201700030000236. [ Links ]
37. Soriano-Melgar L de AA, Izquierdo-Oviedo H, Saucedo-Espinosa YA, Cárdenas-Flores A. Efecto de la aplicación de bioestimulantes sobre la calidad y capacidad antioxidante de frutos de calabacita (Cucurbita pepo37. L. var.' Grey Zucchini'). Terra Latinoamericana [Internet]. 2020;38(1):17-28. Available from: http://www.scielo.org.mx/scielo.php?pid=S0187-57792020000100017&script=sci_arttext37. [ Links ]
38. González Gómez LG, Jiménez Arteaga MC, Castillo Cruz D, Paz Martínez I, Cambara Rodríguez AY, Falcón Rodríguez A, et al. Respuesta agronómica del pepino a la aplicación de QuitoMax en condiciones de organoponía. Centro Agrícola [Internet]. 2018 [cited 24/08/2021];45(3):27-31. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0253-57852018000300027&lng=es&nrm=iso&tlng=en38. [ Links ]
39. Alvarez Pinedo A, Calderón Puig AA, Fundora Sánchez LR, Rodríguez Fajardo A. Manejo de bioproductos en el cultivo del pimiento (Capsicum annuum39. L.) en condiciones de organopónico Management of bioproducts in the pepper cultivation (Capsicum annuum39. L.) under organoponic conditions. Agroecosistemas. 2018;6(2):121-7. Available from: https://aes.ucf.edu.cu/index.php/aes/article/view/20139. [ Links ]
40. Khan WM, Prithiviraj B, Smith DL. Effect of Foliar Application of Chitin and Chitosan Oligosaccharides on Photosynthesis of Maize and Soybean. Photosynthetica [Internet]. 2002 [cited 24/08/2021];40(4):621-4. doi:10.1023/A:1024320606812 [ Links ]
41. Costales-Menéndez D, Falcón-Rodríguez AB. Combinación de formas de aplicación de quitosano en el desarrollo de soya biofertilizada. Cultivos Tropicales [Internet]. 2018 [cited 24/08/2021];39(3):71-9. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362018000300010&lng=es&nrm=iso&tlng=es41. [ Links ]
42. Rodríguez LM, Gómez GG, Arteaga MCJ. Evaluación de productos bioactivos en semilleros en bandejas en el cultivo del pimiento (Capsicum annum42. , L.) (Original). Redel. Revista Granmense de Desarrollo Local. 2019;3(2):220-30. [ Links ]
43. Peil RM, Galvez JL. Dry-matter partitioning as a determinant of greenhouse fruit vegetable crops production. Revista Brasileira de Agrociencia [Internet]. 2005 [cited 24/08/2021];11(1):5-11. Available from: https://eurekamag.com/research/013/053/013053111.php43. [ Links ]
44. Krzesinski W, Knaflewski M. Preliminary model of carrot growth. Acta Horticulturae [Internet]. 2004;654:235-42. doi:10.17660/ActaHortic.2004.654.26 [ Links ]
45. Hole CC, Barnes A, Thomas TH, Scott PA, Rankin WEF. Dry Matter Distribution between the Shoot and Storage Root of Carrot (Daucus carota45. L.): I. Comparison of Varieties. Annals of Botany [Internet]. 1983 [cited 24/08/2021];51(2):175-87. doi:10.1093/oxfordjournals.aob.a086456 [ Links ]
46. Jiao Z, Li Y, Li J, Xu X, Li H, Lu D, et al. Effects of Exogenous Chitosan on Physiological Characteristics of Potato Seedlings Under Drought Stress and Rehydration. Potato Research [Internet]. 2012 [cited 24/08/2021];55(3):293-301. doi:10.1007/s11540-012-9223-8 [ Links ]
47. Jerez Mompié E, Martín Martín R, Morales Guevara D. Comportamiento de la acumulación y distribución de masa seca en tres variedades de papa (Solanum tuberosum47. L.). Cultivos Tropicales [Internet]. 2015 [cited 24/08/2021];36(4):70-6. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362015000400009&lng=es&nrm=iso&tlng=es47. [ Links ]
48. Jerez Mompie EI, Martín Martín R, Morales Guevara D, Díaz Hernández Y. Análisis clásico del crecimiento en tres variedades de papa (Solanum tuberosum48. L.). Cultivos Tropicales [Internet]. 2016 [cited 24/08/2021];37(2):79-87. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362016000200009&lng=es&nrm=iso&tlng=en48. [ Links ]
49. Hernández N, Soto F. Influencia de tres fechas de siembra sobre el crecimiento y la relación fuente- demanda del cultivo del maíz (Zea mays49. L.). Cultivos Tropicales [Internet]. 2012 [cited 24/08/2021];33(1):28-34. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362012000100004&lng=es&nrm=iso&tlng=es49. [ Links ]
50. Gómez LGG, Arteaga MCJ, Pizarro MJ, Lahera AO, Zayas AA, Rodriguez AF. Evaluación del Pectimorf(r) y Quitomax(r) en el cultivo de la papaya (Carica papaya50. , L) cv Maradol roja (Original). Redel. Revista granmense de Desarrollo Local. 2019;3(4):262-71. [ Links ]
51. Rodríguez-Pedroso AT, Ramírez-Arrebato MÁ, Falcón-Rodríguez A, Bautista-Baños S, Ventura-Zapata E, Valle-Fernández Y. Efecto del Quitomax(r) en el rendimiento y sus componentes del cultivar de arroz (Oryza sativa51. L.) var. INCA LP 5. Cultivos Tropicales [Internet]. 2017 [cited 24/08/2021];38(4):156-9. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362017000400002&lng=es&nrm=iso&tlng=pt51. [ Links ]
52. Jiménez Arteaga MC, González Gómez LG, Verdecia Corría AJ, Oliva Lahera A, Jiménez Arteaga MC, González Gómez LG, et al. Respuesta de Phaseolus vulgaris a la aplicación de Azofert-F, Micorriza y QuitoMax a la semilla, en dos períodos de siembra. Centro Agrícola [Internet]. 2020 [cited 24/08/2021];47(1):22-7. Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0253-57852020000100022&lng=es&nrm=iso&tlng=pt52. [ Links ]
53. Sopalo WIL, Gómez LGG, Fabré TB. Comportamiento del tomate (Solanum lycopersicum53. L.) variedad Amalia en Cuba y Ecuador al aplicarle QuitoMax. Redel. Revista Granmense de Desarrollo Local. 2020;4:515-26. [ Links ]
54. Martínez IP, Gómez LGG, Fabré TB, Arteaga MCJ. Evaluación de algunos indicadores de calidad y rendimiento en las variedades de tomate ESEN y L-43, al aplicarle QuitoMax. Redel. Revista Granmense de Desarrollo Local [Internet]. 2020;4:541-51. Available from: http://docplayer.es/205734011-Original-evaluacion-de-algunos-indicadores-de-calidad-y-rendimiento-en-las-variedades-de-tomate-esen-y-l-43-al-aplicarle-quitomax.html54. [ Links ]
Received: August 15, 2020; Accepted: March 22, 2021















